Radiology
State-of-the-art imaging enables precise diagnoses and optimal treatment planning. Radiology at Helios combines high technology with medical expertise.
An overview of key financial figures is available at the end of the release.
1 Before special items
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation
3 Growth rate adjusted for Argentina hyperinflation
4 Excluding Fresenius Medical Care
5 At average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures, including lease liabilities, including Fresenius Medical Care dividend, net debt adjusted for the valuation effect of the equity-neutral exchangeable bond
"Fresenius is accelerating with purpose, and our transformation is delivering tangible results. Our disciplined execution and performance-oriented culture have resulted in 14% growth in Core EPS, a 6% increase in organic revenue growth, and margin improvements, enabling us to raise our full-year EBIT guidance to 4%-8%.
Fresenius Kabi and Fresenius Helios continue to perform well, while investments in digital health and advanced therapies are reshaping patient care. Despite the current macroeconomic environment, we continue to perform and meet our commitments. With Rejuvenate now driving measurable progress and a clear focus on patient care, we are creating sustainable value for patients, partners, and shareholders – today and into the future."
Based on the strong earnings growth in Q1-3/2025, Fresenius raised the Group EBIT growth guidance:
Fresenius Group2: organic revenue growth3 in the range of 5 to 7%; constant currency EBIT growth4 now expected in the range of 4% to 8% (previous: 3 to 7%)
Fresenius Kabi5: organic revenue growth3 in the mid- to high-single-digit percentage range; EBIT margin of 16.0% to 16.5%
Fresenius Helios6: organic revenue growth in the mid-single-digit percentage range; EBIT margin around 10%
The strong performance in Q1-3/2025 gives the scope to deliberately take some investments in Q4/2025, for example in R&D. This is in line with Fresenius’ strategic roadmap for the Rejuvenate phase to upgrade core and scale platforms and with a view on future performance, i.e. investing in further long-term profitable growth.
1 Before special items
2 2024 base: €21,526 million (revenue) and €2,489 million (EBIT)
3 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation
4 Growth rate adjusted for Argentina hyperinflation
5 2024 base: €8,414 million (revenue) and €1,319 million (EBIT)
6 2024 base: €12,739 million (revenue) and €1,288 million (EBIT)
Assumptions to guidance: When Fresenius gave guidance in February 2025, the company acknowledged the fast-moving macro-economic and geopolitical environment, resulting in a higher level of operational uncertainty. Fresenius’ guidance continues to reflect current factors and known uncertainties such as impacts from tariffs to the extend they can currently be assessed. The guidance does not take into account potential extreme scenarios that could affect the company, its peers, and the healthcare sector as a whole.
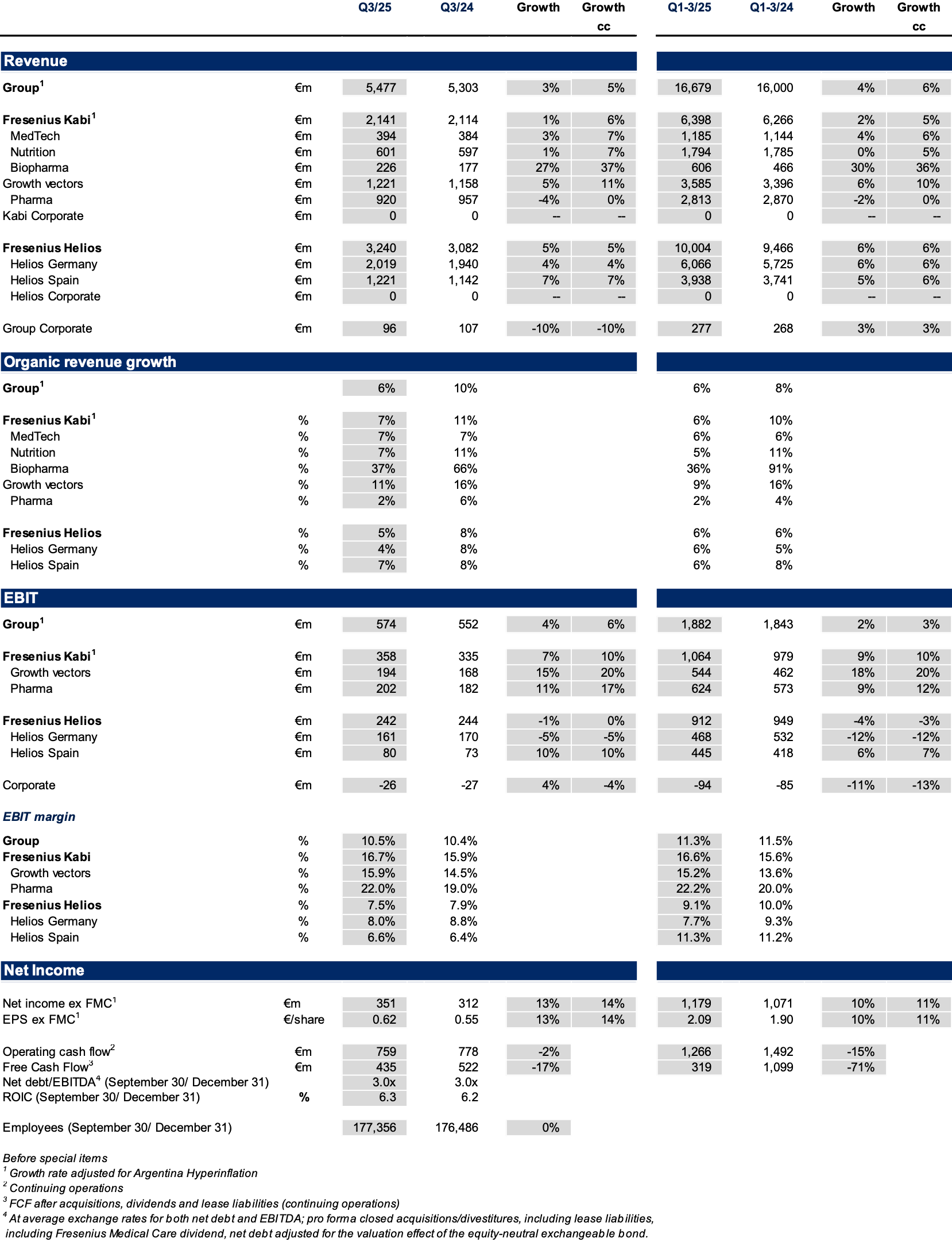
In Q3/2025, the strong operating performance at Fresenius Kabi, and solid development at Fresenius Helios led to consistent Group organic revenue1 growth of 6%2 with revenues reaching €5,477 million.
Group EBIT before special items amounted to €574 million, a sequential acceleration with an increase of 6%3 in constant currency despite the absence of energy relief payments at Helios Germany, the usual seasonality at the Spanish hospital business in the third quarter, and the impact of the Volume Based Procurement of the nutrition product Ketosteril in China at Fresenius Kabi. Group EBIT margin1 improved to 10.5% (Q3/24: 10.4%). The Helios Performance Programme is advancing with material contributions expected in Q4/2025 with likely some spill-over into 2026. The strong Q3/2025 EBIT development was additionally supported by positive phasing effects.
Group net income1,4 increased by excellent 14%3 in constant currency to €351 million strongly outpacing revenue growth. The good operating performance of the core businesses, further productivity gains at Fresenius Kabi and strict cost discipline at Fresenius Helios drove this strong performance, and was supported by the significantly decreased year-over-year interest expenses. In the third quarter alone, interest expenses decreased by €35 million.
Core Earnings per share1,4 rose by excellent 14%3 in constant currency to €0.62.
1 Before special items
2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation
3 Growth rate adjusted for Argentina hyperinflation
4 Excluding Fresenius Medical Care
Fresenius Kabi showed strong organic growth at the upper end of the structural growth range with Growth Vectors driving the performance, headed by continued Biopharma strength; EBIT margin above the 2025 guidance range supported by productivity gains and some contributions realized earlier than originally expected.
Organic revenue growth of 7%1 driven by the Growth Vectors, also benefitting from some inflation related pricing effects in Argentina; disciplined execution on product rollouts led to contributions already realized in Q3/2025 that were originally expected for Q4; revenue increased to €2,141 million; growth as reported was negatively impacted by currency effects; increase in constant currency of 6%2.
1 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation
2 Growth rate adjusted for Argentina hyperinflation
3 Before special items
Strong 5% organic revenue growth driven by year-over-year activity levels increase at both, Helios Germany and Helios Spain; revenue increased by 5% in constant currency to €3,240 million.
1 Before special items
As part of the publication of the Q3/2025 results, a conference call will be held on November 5, 2025 at 1:30 p.m. CEST / 7:30 a.m. EDT. All investors are cordially invited to follow the conference call in a live audio webcast at https://www.fresenius.com/investors. Following the call, a replay will be available on our website.

This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
State-of-the-art imaging enables precise diagnoses and optimal treatment planning. Radiology at Helios combines high technology with medical expertise.
With state-of-the-art robot technology, the DaVinci surgical robot enables particularly gentle and precise procedures.
Fresenius is a global healthcare company headquartered in Bad Homburg v. d. Höhe, Germany. The Fresenius Group comprises the operating companies Fresenius Kabi and Fresenius Helios as well as an investment in Fresenius Medical Care.
Fresenius is a global healthcare company headquartered in Bad Homburg v. d. Höhe, Germany. The Fresenius Group comprises the operating companies Fresenius Kabi and Fresenius Helios as well as an investment in Fresenius Medical Care.
Fresenius is a global healthcare company headquartered in Bad Homburg v. d. Höhe, Germany. The Fresenius Group comprises the operating companies Fresenius Kabi and Fresenius Helios as well as an investment in Fresenius Medical Care.
November 13, 2025
virtual
Event for retail investors (German only)