Investors
#FutureFresenius: REJUVENATE in Action – Delivering accelerated performance for long-term value creation
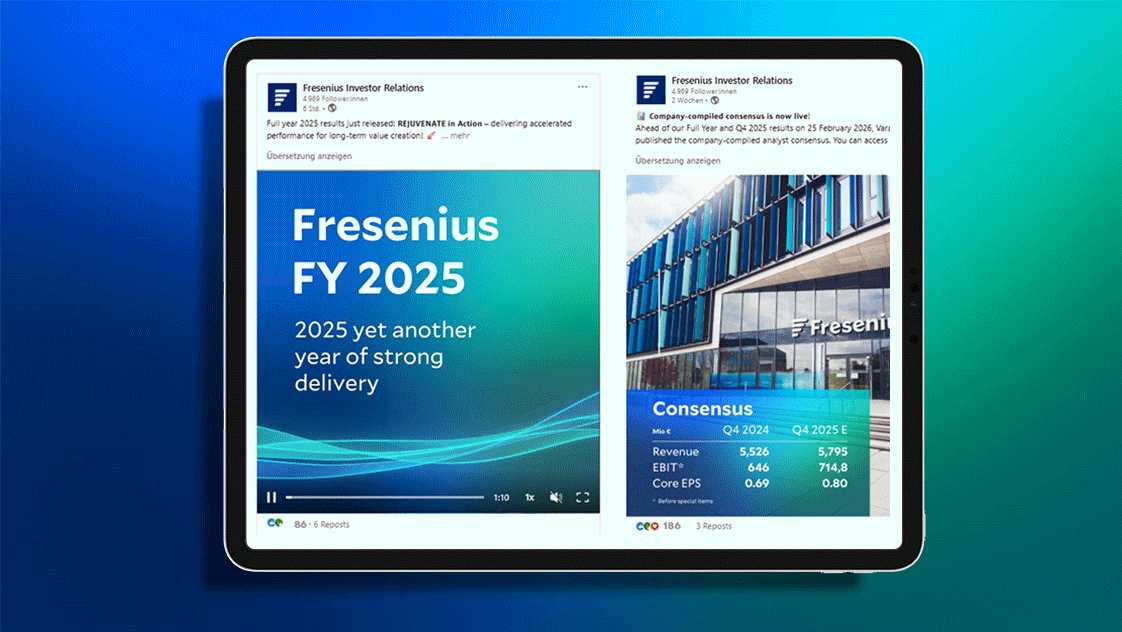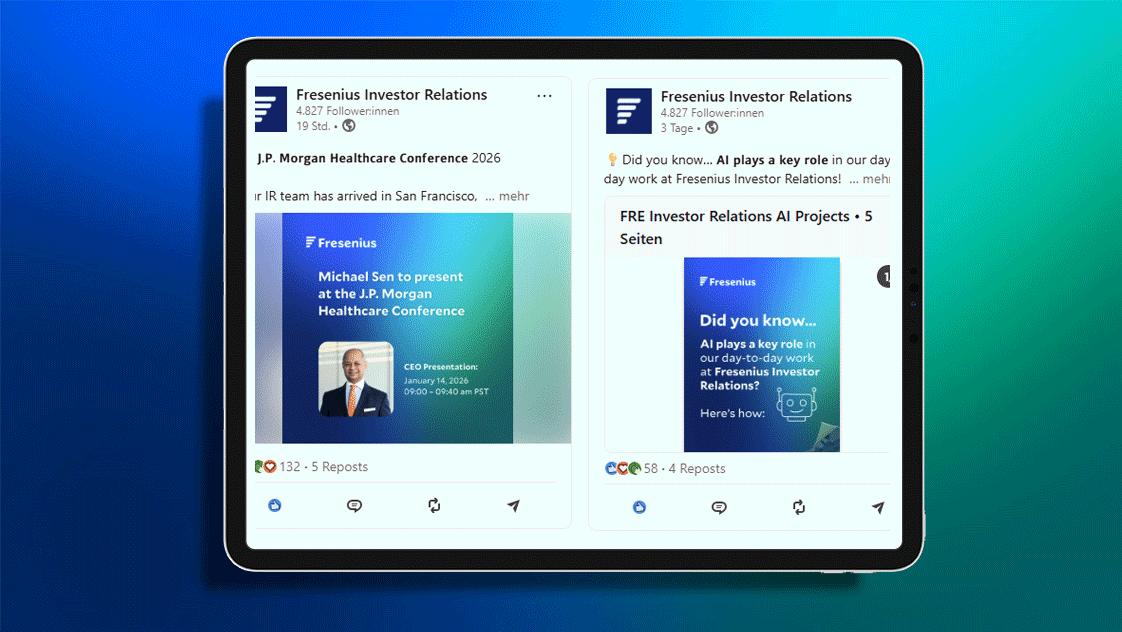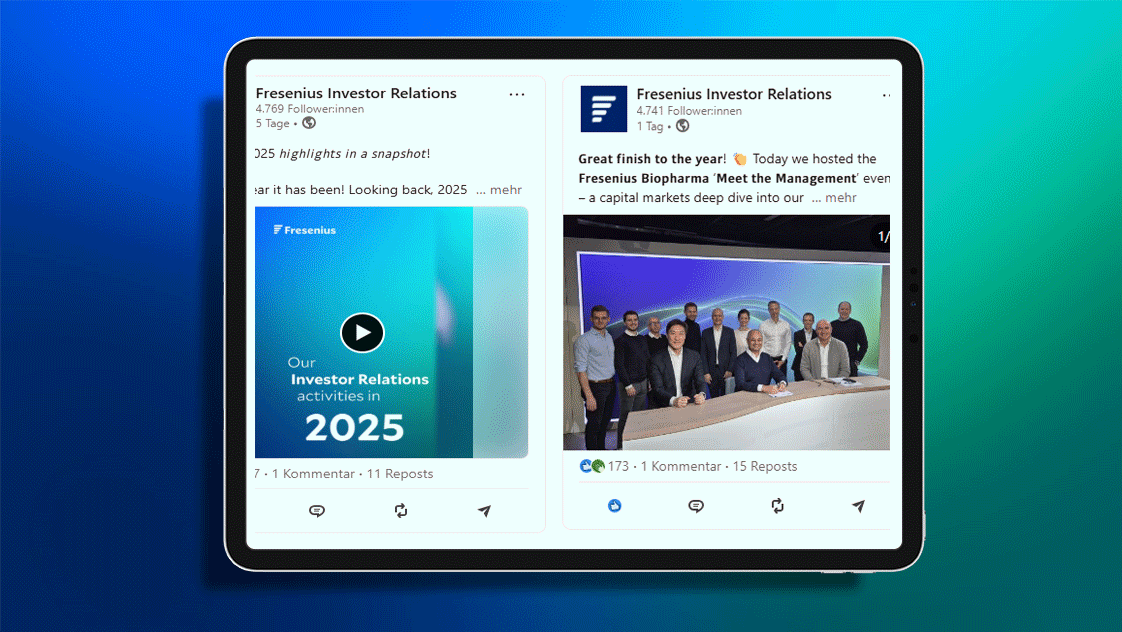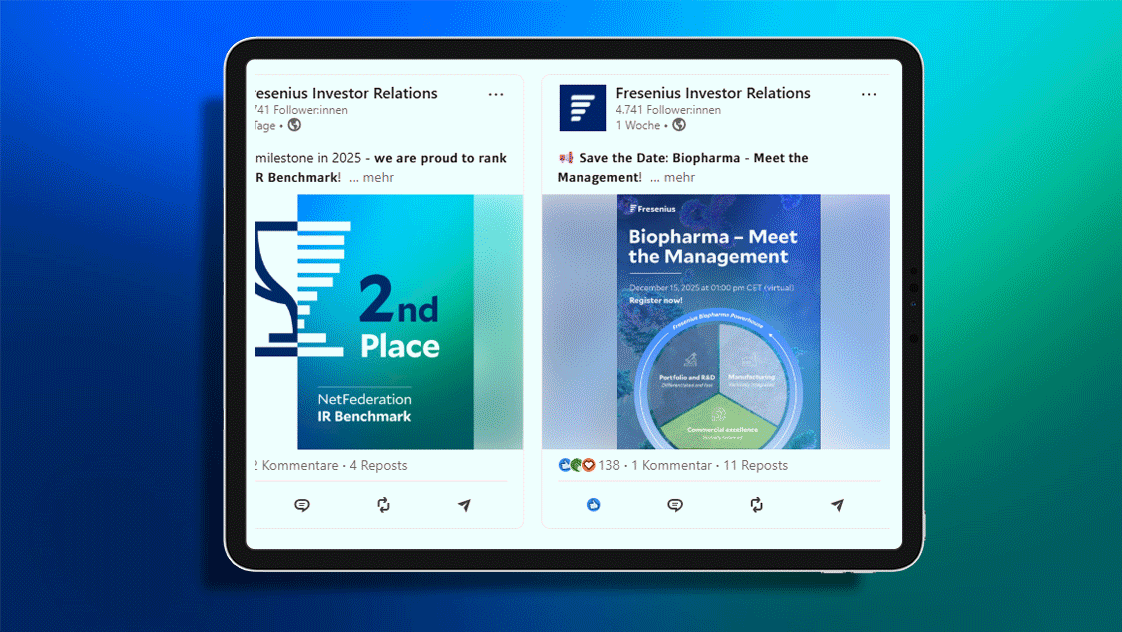
FY/25: Strong organic revenue and excellent Core EPS growth
- Organic revenue growth of 7%1,2, reflecting the consistent execution across Fresenius Kabi and Fresenius Helios
- Group EBIT1 up 6%3 in constant currency, driven in particular by Fresenius Kabi’s Growth Vectors and the strong performance of Fresenius Helios in Spain
- Core EPS1,4 increased 12%3 in constant currency based on strong operating results and significantly decreased interest expense
- Structural EBIT margin ambition increased for Fresenius Kabi to 17 to 19% (previously 16 to 18%)
- Constant currency Core EPS growth1,4 established as new guidance metric is expected to be in the range of 5 to 10%3 in FY/26; organic revenue growth2 projected to be in the range of 4 to 7% in FY/26.
- Dividend proposal of €1.05 per share; year-on-year increase of 5%, in line with the Company's dividend payout ratio of 30 to 40%.
1 Before special items; 2 Organic growth rate adjusted for accounting effects related to Argentina hyperinflation; 3 Growth rate adjusted for Argentina hyperinflation; 4 Excluding Fresenius Medical Care
View the full FY/25 update in the Financial Results section.
Read more about our company strategy!

February 25, 2026 - 01:30 pm
Bad Homburg, Germany
Conference Call Full Year Results 2025

"2025 was a pivotal year for Fresenius. With disciplined execution of our #FutureFresenius strategy and a strong performance from Team Fresenius, we met our upgraded full-year guidance by delivering another quarter of competitive growth [...]. 2025 capped a year of continued momentum across the Company: We further strengthened the balance sheet, and upgraded our guidance, while preparing the business through targeted investment for the next phase of growth. All of this leads to a proposed dividend of €1.05 per share, underscoring our commitment to creating shareholder value [...]. With #FutureFresenius we have transformed our Company, positioning ourselves to deliver future success in a new world order. Looking ahead, we enter 2026 with strong foundations and clear priorities [...]."
Michael Sen, Chairman of the Management Board
Financial Results
FY 2025: REJUVENATE in Action – Delivering accelerated performance for long-term value creation!
Outlook
New guidance metric established: Fresenius Outlook 2026 at a glance.
Financial Reports
Discover Fresenius financial reports and presentations here!
Goals & Strategy
#FutureFresenius is clearly paying-off. Find out more about our company's strategy!

Investor Feedback
Open dialogue is valuable for us.
You can submit your feedback quickly and easily using the link below (anonymously if you prefer).
You will be redirected to QuantiFire which conducts this survey in own controllership.
Stay Connected with Fresenius Investor Relations on LinkedIn
Follow Fresenius Investor Relations for expert insights and latest news that contribute to the Fresenius equity story, and exclusive content from our team. Join our growing community and be part of the conversation!