Fresenius AG today announced that the share split with capital increase from the Company's funds approved by the Extraordinary General Meeting on December 4, 2006, will become effective on February 2, 2007. On the same day, the shares will be traded "ex split" and the shareholders' deposits will be adapted to the new number of shares. Every holder of an ordinary share now holds three ordinary shares and every holder of a preference share holds three preference shares.
The Fresenius shares will continue to trade under ISIN DE0005785604 (ordinary share) and ISIN DE0005785638 (preference share).
The subscribed capital of Fresenius AG now amounts to € 154.4 million, divided into 77,176,938 ordinary shares and 77,176,938 preference shares.
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and the ambulatory medical care of patients. In 2006 group sales are expected to increase to more than € 10.7 billion. On September 30, 2006 the Fresenius Group had 104,179 employees worldwide.
Fresenius Medical Care, the world's leading renal therapy company, today announced that is has agreed with Amgen Europe (Amgen) to establish a joint research project aimed at further improving treatment outcomes for kidney patients in Europe.
In the scope of the joint research agreement, Fresenius Medical Care and Amgen will facilitate a working group of European scientific experts in the renal field. The working group will analyze practices in the treatment of chronic kidney disease (CKD) and will publish their findings for improved therapeutic options.
The working group will be led by renowned independent scientists in the field of nephrology, who will lead the analysis process. Both Amgen and Fresenius Medical Care will engage renowned consultants in the field of nephrology who will help lead the analysis process and develop additional research questions.
The focus of the first research efforts will be on anemia and bone mineral disease affecting patients with kidney failure.
Dr. Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "We are pleased to partner with Amgen in this European initiative. This agreement is a pure research project designed to pool both companies' expertise in the field and analyze data available. This is yet another step in our long-term growth strategy to further enhance our renal drug initiative. Our objective is to assist further improvements in patient medical outcomes with our customers and our own network of clinics."
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,400,000 individuals worldwide. Through its network of 2,085 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 161,433 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS-p).
For more information about Fresenius Medical Care visit the website: www.fmc-ag.com.
About Amgen
Amgen discovers, develops and delivers innovative human therapeutics. A biotechnology pioneer since 1980, Amgen was one of the first companies to realize the new science's promise by bringing safe and effective medicines from lab, to manufacturing plant, to patient. Amgen therapeutics have changed the practice of medicine, helping millions of people around the world in the fight against cancer, kidney disease, rheumatoid arthritis, and other serious illnesses. With a broad and deep pipeline of potential new medicines, Amgen remains committed to advancing science to dramatically improve people's lives.
For more information about Amgen visit the Company's website at www.amgen.com.
Fresenius Medical Care, the world's leading renal therapy company, today announced that it has entered into a new agreement with Amgen Europe (Amgen) for Aranesp (darbepoetin alfa).
Under the agreement, Fresenius Medical Care will devote efforts to assist Amgen in disseminating scientific information regarding the treatment of anemia to nephrologists and other dialysis experts. Amgen and its representatives are and will remain solely responsible for the product. The new agreement runs for three years.
Patients with chronic kidney disease on dialysis and not on dialysis often suffer from complications such as anaemia, which occurs when failing kidneys no longer produce sufficient erythropoietin, a hormone that stimulates the production of oxygen-carrying red blood cells. Aranesp is a novel recombinant erythropoiesis stimulating protein (ESP) that stimulates erythropoiesis to produce red blood cells. Aranesp has a proven tolerability and proven efficacy, across all stages of chronic kidney disease.
Dr. Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "We are pleased to partner with Amgen on an expanded scale to further improve medical outcomes for dialysis patients in Europe. Amgen and Fresenius Medical Care are well known for their commitment to developing and establishing new therapies for the benefit of patients with endstage renal disease. Furthermore, this agreement clearly advances our renal drug initiatives and emphasizes the position of both companies as leaders in nephrology innovation."
# # #
Aranesp is a trademark of Amgen Inc.
Aranesp Summary of Product Characteristics. European Medicines Agency.
www.emea.eu.int/humandocs/Humans/EPAR/aranesp/aranesp.htm (Accessed 28th November 2006)
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,400,000 individuals worldwide. Through its network of 2,085 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 161,433 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS-p).
For more information about Fresenius Medical Care visit the website: www.fmc-ag.com.
About Amgen
Amgen discovers, develops and delivers innovative human therapeutics. A biotechnology pioneer since 1980, Amgen was one of the first companies to realize the new science's promise by bringing safe and effective medicines from lab, to manufacturing plant, to patient. Amgen therapeutics have changed the practice of medicine, helping millions of people around the world in the fight against cancer, kidney disease, rheumatoid arthritis, and other serious illnesses. With a broad and deep pipeline of potential new medicines, Amgen remains committed to advancing science to dramatically improve people's lives.
For more information about Amgen visit the Company's website at www.amgen.com.
Summary Full Year 2006:
The Company exceeded its financial targets, achieved record earnings and proposes its 10th consecutive annual dividend increase.
Net revenue : $ 8,499 million, + 26%
Operating income (EBIT): $ 1,318 million, + 40%
Net income: $ 537 million, + 18%
Excluding SFAS 123(R) and one-time items
Operating income (EBIT): $ 1,329 million, + 38%
Net income: $ 584 million, + 24%
Dividend proposal
Ordinary share: € 1.41, + 15%
Preference share: € 1.47, + 14%
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced its results for the fourth quarter and full year of 2006.
Please note, the result of operations of Renal Care Group (RCG) are consolidated from April 1, 2006 onward.
Fourth Quarter 2006:
Revenue
Net revenue for the fourth quarter 2006 compared to the fourth quarter 2005 increased by 33% (31% at constant currency) to $2,352 million. Organic revenue growth worldwide was 11%. Dialysis Services revenue grew by 39% to $1,749 million (38% at constant currency) in the fourth quarter of 2006. Dialysis Product revenue increased by 17% to $603 million (12% at constant currency) in the same period.
North America revenue increased by 39% to $1,658 million. Dialysis Services revenue increased by 44% to $1,505 million. Average revenue per treatment for the U.S. clinics increased by 9% to $328 in the fourth quarter 2006 compared to $302 for the same quarter in 2005. Dialysis Product revenue increased by 5% to $153 million led by strong sales of our 2008K hemodialysis machines. Excluding the effects of the RCG acquisition and related divestitures, the Dialysis Product revenue increased by 13% compared to last year.
International revenue was $694 million, an increase of 20% (14% at constant currency) compared to the fourth quarter of 2005. Dialysis Services revenue reached $244 million, an increase of 17% (13% at constant currency). Dialysis Product revenue increased by 22% to $450 million (15% at constant currency), led by strong sales of machines (both the 4008 and 5008 series), dialyzers and peritoneal dialysis products.
Earnings
Operating income (EBIT) increased by 45% to $354 million. Operating income for the fourth quarter 2006 includes $29 million of costs related to the change of accounting principles for stock options (SFAS 123R), restructuring costs and in-process R&D.
Excluding these costs, operating income for the fourth quarter 2006 increased by 48% to $383 million resulting in an operating margin of 16.3%. For the fourth quarter 2005 the operating margin was 14.6%.
Compared with the fourth quarter 2005, the operating margin excluding one-time items in North America increased by 260 basis points to 17.1% due to the consolidation of RCG, an increase in the revenue per treatment and strong demand for dialysis products. In the International segment, the operating margin increased from an already high level by 30 basis points to 17.7%. The continued strong operational performance in the International segment was driven by increased product sales in all regions and positively impacted by production efficiencies and reimbursement increases in key countries in Europe and Latin America.
Net interest expense for the fourth quarter 2006 was $96 million compared to $46 million in the same quarter of 2005. This increase is entirely attributable to the debt financing for the RCG acquisition.
Income tax expense was $99 million for the fourth quarter of 2006 compared to $82 million in the fourth quarter of 2005, reflecting effective tax rates of 38.5% and 41.3%, respectively.
Net income for the fourth quarter 2006 was $152 million, an increase of 32%. Excluding one-time costs and SFAS 123(R), the net income increased on a comparable basis by 35% to $172 million.
Earnings per share (EPS) for the fourth quarter of 2006 rose by 31% to $1.55 per ordinary share ($0.52 per American Depositary Share (ADS)) compared to $1.18 ($0.39 per ADS) for the fourth quarter of 2005. The weighted average number of shares outstanding for the fourth quarter of 2006 was approximately 98.3 million shares compared to 97.6 million shares for the fourth quarter of 2005. The increase in shares outstanding results from stock option exercises in 2006.
Cash Flow
In the fourth quarter of 2006, the Company generated $443 million in cash from operations, representing 19% of revenue – clearly above our target. The strong cash flow generation was supported by increased earnings.
A total of $177 million was spent for capital expenditures, net of disposals. Free Cash Flow before acquisitions was $266 million compared to $65 million in the fourth quarter of 2005. A total of $118 million in cash was used for acquisitions.
Full Year 2006:
Earnings and Revenue
For the full year 2006, net income was $537 million, up 18% from 2005. Excluding costs related to the change of accounting principles for stock options (SFAS 123R) of $10 million and one-time items of $37 million, net income increased by 24% to $584 million. In the future, costs related to SFAS 123(R) will not be adjusted when comparing with 2007 figures.
Net revenue for 2006 was $8,499 million, up 26% from 2005. Adjusted for currency, net revenue rose by 25%. Organic growth was 10%.
Operating income (EBIT) increased by 40% to $1,318 million. Operating income for the full year 2006 includes expenses of $11 million as a result of restructuring and in-process R&D, transformation of legal form and the change of accounting principles for stock options, net of the gain from the clinic divestitures.
Excluding these items and the one-time costs for the transformation of the legal form in the prior year, operating income for 2006 increased by 38% to $1,329 million. This performance resulted in an operating margin of 15.6% compared to 14.2% for the year 2005.
Net interest expense for the full year 2006 was $351 million. The increase was the result of three quarters worth of additional interest expense and the write-off of deferred financing costs related to the 2003 senior credit facility of $15 million, both in conjunction with the financing of the RCG acquisition.
Income tax expense was $413 million for the full year compared to $309 million in 2005, reflecting tax rates of 42.8% and 40.3%, respectively. The tax rate was impacted by tax payments in connection with the gain on divestiture of dialysis clinics in the U.S. and by a tax audit in Germany. Excluding these impacts, the effective tax rate was 38.5%.
For the full year 2006, earnings per ordinary share rose by 17% to $5.47 ($1.82 per ADS). The weighted average number of shares outstanding during 2006 was approximately 98.1 million.
Cash Flow
Cash from operations during the full year 2006 was $908 million compared to $670 million for 2005 on a reported basis. On an adjusted basis, cash from operations was $1,106 million in 2006 compared to $805 million in 2005. The increase compared to prior the year was mainly due to increased earnings and further improvements in working capital efficiency.
A total of $450 million was used for capital expenditures, net of disposals. Free Cash Flow before acquisitions for 2006 was $458 million compared to $373 million in 2005. The underlying Free Cash Flow before acquisitions and one-time effects for 2006 was $656 million. A total of $159 million in cash was used for acquisitions other than the RCG acquisition in 2006.
For a complete overview on the fourth quarter and the full year of 2006 and the reconciliation of non-GAAP financial measures included in this release to the most comparable GAAP financial measures, please refer to the attachments.
Patients – Clinics – Treatments
As of December 31, 2006, Fresenius Medical Care treated 163,517 patients worldwide, which represents a 24% increase in patients compared to last year. North America provided dialysis treatments for 117,855 patients (up 32%). Including 33 managed clinics, the number of patients in North America was 119,883. The International segment served 45,662 patients (up 8%).
As of December 31, 2006, the Company operated a total of 2,108 clinics worldwide. This is comprised of 1,560 clinics in North America, an increase of 35%, and 548 clinics in the International segment, an increase of 5%.
Fresenius Medical Care delivered approximately 23.74 million dialysis treatments worldwide, which represents an increase of 20% year over year. North America accounted for 16.88 million treatments, an increase of 25%, and the International segment delivered 6.86 million treatments, an increase of 10% over last year.
Employees
As of December 31, 2006, Fresenius Medical Care employed 56,803 people (full-time equivalents) worldwide compared to 47,521 at the end of 2005. The increase of 9,282 employees is primarily due to the acquisition of Renal Care Group.
Dividends
The Company will continue to follow an earnings-driven dividend policy. For the tenth consecutive year, shareholders can expect to receive an increased annual dividend for the fiscal year 2006. At the Annual General Meeting to be held on May 15, 2007, shareholders will be asked to approve a dividend of €1.41 per ordinary share, an increase of 15% from 2005 (€1.23) and €1.47 per preference share, an increase of 14% from 2005 (€1.29).
Acquisition in Taiwan
On January 9, 2007, the Company announced the acquisition of a 51% equity interest in Jiate Excelsior Co. Ltd. ("Excelsior") in Taiwan. The purchase price for the 51% equity interest in Excelsior was $38 million. The transaction is expected to add about $80 million to the consolidated revenues and to be accretive to the Company's earnings for 2007. Through the transaction, Fresenius Medical Care will become the leading dialysis provider in the Asia-Pacific region.
Outlook for 2007
For the full year 2007, the Company expects to achieve revenue of approximately $9.4 billion, an increase of 11%.
The net income is expected to be between $675 million and $695 million in 2007. This represents an increase of between 18% and 21% on an adjusted basis to 2006. On a reported basis, the net income would increase by 26% to 29%.
The Company expects spending on capital expenditures and acquisitions to be at approximately $650 million. The debt/EBITDA ratio is expected to fall below 3.0 by the end of 2007.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "Our excellent financial performance for the year reflects the strong demand for our products and services worldwide. We are pleased to have again delivered on our commitments in 2006 and are proposing to deliver our tenth consecutive dividend increase to our shareholders. With the successful integration of the Renal Care Group acquisition, expansion opportunities for patient care in Europe and Asia and the progress on our growth initiatives, we are confident as we look ahead."
Video Webcast
Fresenius Medical Care will hold a press conference at its headquarters in Bad Homburg, Germany, to discuss the results of the fourth quarter and the full year of 2006 on February 22, 2007, at 10:00 am CET. The Company cordially invites journalists to view the live video webcast of the meeting at this website. A replay will be available shortly after the meeting.
# # #
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects about 1,500,000 individuals worldwide. Through its network of 2,108 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 163,517 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS-p).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Fresenius Medical Care
Statement of Earnings
see pdf-file
- Sales € 10.8 billion, + 37 % at actual rates, + 37 % in constant currency
- EBIT € 1,444 million, + 49 % at actual rates, + 50 % in constant currency
- Net income € 330 million, + 49 % at actual rates, + 49 % in constant currency
- Sales exceeding the € 10 billion mark and EBIT exceeding the € 1 billion mark for the first time
- Excellent sales and earnings growth in all business segments
- Further margin improvements in all business segments achieved
- 15 % increase in dividend per share proposed
- Strong cash flow supports rapid de-levering
14th consecutive dividend increase proposed
2006 was the most successful year in the Company's history. Based on the Group's excellent financial results, the Management Board will propose to the Supervisory Board a dividend increase of 15 % to € 0.57 per ordinary share (2005, adjusted for the share split: € 0.49) and to € 0.58 per preference share (2005, adjusted for the share split: € 0.50). The total dividend distribution is expected to be € 88.8 million (2005: € 75.8 million).
Positive outlook for 2007
For 2007, Fresenius Group projects further improvements in sales and earnings. Group sales are expected to grow by 8 to 10 % in constant currency. Net income is expected to increase by 20 to 25 % in constant currency. We anticipate further margin improvements of all business segments to contribute to this growth.
Investments in property, plant and equipment and in intangible assets are planned to increase from € 600 million in 2006 to € 600 to 700 million.
Strong sales growth across all business segments and regions
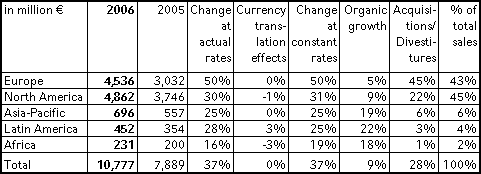
Group sales increased by 37 % to € 10,777 million in 2006 (2005: € 7,889 million). Organic growth was 9 %. Acquisitions, in particular Renal Care Group and HELIOS Kliniken, contributed 29 %. Divestitures had a -1 % effect on sales. Currency translation effects had no impact.
Strong sales growth was achieved in the core markets of North America and Europe. In North America, sales grew significantly due to the Renal Care Group consolidation and an excellent organic growth rate of 9 %. In Europe, the substantial sales increase was mainly driven by the consolidation of HELIOS Kliniken. Organic growth in Europe was 5 %. Excellent growth rates were achieved in the emerging markets with organic growth of 19 % in Asia-Pacific, 22 % in Latin America and 18 % in Africa.

Sales contribution of the three business segments:


Fresenius ProServe's increased sales contribution is the result of the consolidation of HELIOS Kliniken.
Excellent earnings growth
Group operating income (EBIT) increased by 49 % at actual rates and by 50 % in constant currency to € 1,444 million (2005: € 969 million). The Group EBIT margin improved to 13.4 % (2005: 12.3 %). Operating income for the full year 2006 includes a gain of € 32 million from the divestitures of dialysis clinics in the USA. The sale was a condition of the US Federal Trade Commission for the approval of the Renal Care Group acquisition. Operating income for the full year 2006 also includes a total of € 44 million for one-time expenses, e.g. for the integration of Renal Care Group, as well as for expenses related to the stock option accounting change.
Group net interest was € -395 million (2005: € -203 million). The increase was primarily driven by the debt financing of the Renal Care Group acquisition. Net interest also includes one-time expenses of € 30 million associated with the early refinancing of Group debt.
The tax rate was 39.5 % (2005: 38.9 %). It was substantially influenced by the tax expense associated with the divestiture of the dialysis clinics in the USA as the goodwill attributable to the divested clinics is not considered for tax purposes. Excluding this effect the tax rate was 37.2 %, well within our guidance of 36 to 38 %.
Minority interest was € 305 million (2005: € 246 million), of which 93 % was attributable to the minority interest in Fresenius Medical Care.
Group net income grew strongly by 49 % at actual rates as well as in constant currency to € 330 million (2005: € 222 million). Net income includes a total of € 29 million for one-time expenses, primarily for the early refinancing of debt, for the integration of Renal Care Group, and for expenses related to the stock option accounting change.
Earnings per ordinary share were € 2.15 (2005, adjusted for the share split: € 1.76) and earnings per preference share* were € 2.16 (2005, adjusted for the share split: € 1.77). This is an increase of 22 % for both share classes. The average number of shares grew to 153,006,012 in 2006.
* In accordance with SFAS 128 („Earnings per Share") the calculation of basic and diluted earnings per share for the fiscal years 2006 and 2005 have been adjusted retrospectively by the increased weighted average number of shares outstanding.
Capital expenditure at high level
Fresenius Group spent € 600 million for property, plant and equipment and intangible assets (2005: € 353 million). Acquisition spending increased to € 3,714 million mainly due to the Renal Care Group acquisition (2005: € 1,894 million).
Strong cash flow
Operating cash flow increased by 35 % to € 1,052 million (2005: € 780 million). Key drivers were the strong increase in earnings and further improvements in working capital management. Cash flow before acquisitions and dividends was € 481 million (2005: € 449 million). The acquisition of Renal Care Group was entirely debt-financed.
Solid balance sheet structure
Total assets increased by 30 % to € 15,024 million (December 31, 2005: € 11,594 million). In constant currency, total assets grew by 38 %. The substantial increase is mainly related to the consolidation of the Renal Care Group. Current assets increased by 16 % to € 4,106 million (December 31, 2005: € 3,531 million). Non-current assets were € 10,918 million (December 31, 2005: € 8,063 million), an increase of 35 %.
Shareholders' equity including minority interest grew by 12 % to € 5,728 million (December 31, 2005: € 5,130 million), driven by the very good earnings development. Given the debt financing of the Renal Care Group acquisition, the equity ratio (including minority interest) decreased to 38.1 % (December 31, 2005: 44.2 %).
Group debt increased to € 5,872 million (December 31, 2005: € 3,502 million) due to the financing of the Renal Care Group acquisition.
As of March 31, 2006, following the closing of the Renal Care Group acquisition, the net debt/EBITDA ratio was at 3.5. Given the excellent earnings growth and a strong cash flow the net debt/EBITDA ratio improved significantly to 3.0 as of December 31, 2006 (December 31, 2005: 2.3).
Employees
As of December 31, 2006, the Group had 104,872 employees (December 31, 2005: 91,971). The increase of 12,901 employees is primarily due to the acquisitions of Renal Care Group and HUMAINE Kliniken.
Fresenius Biotech
Fresenius Biotech develops innovative therapies with trifunctional antibodies for the treatment of cancer as well as cell therapies for the treatment of the immune system. In the field of polyclonal antibodies, Fresenius Biotech has successfully marketed ATG-Fresenius S for many years. ATG-Fresenius S is an immunosuppressive agent used to prevent and treat graft rejection following organ transplantation.
In December 2006, first results of a phase II/III pivotal study on malignant ascites in patients with ovarian cancer were published, including treatment data of 129 patients. The results showed a clear advantage of the therapy with the trifunctional antibody removab® over a therapy with puncture alone. The results of 128 patients with tumor diseases other than ovarian cancer (e.g. gastric cancer) are expected for the first quarter of 2007. Data on overall survival of all patients of the study are expected in the second quarter of 2007. The application dossier for marketing authorization for removab® in malignant ascites is planned to be submitted to the European Medicines Agency (EMEA) in the second half of 2007.
In June 2006, Fresenius Biotech published the results of a phase Ila study in the treatment of ovarian cancer patients assessing tolerability, dose regimen and efficacy. Based on the encouraging results, the company plans to start a phase II study in this indication in Europe in the first half of 2007.
The phase II study with the antibody rexomun® to treat breast cancer, which started in March 2006, and the phase II study with the antibody removab® to treat gastric cancer, which started in June 2006, are ongoing.
Fresenius Biotech's operating income (EBIT) was € -45 million in 2006 (2005: € -41 million). For 2007, Fresenius Biotech expects an EBIT of approximately € -50 million.
The Business Segments
Fresenius Medical Care
Fresenius Medical Care is the world's leading provider of products and services for patients with chronic kidney failure. As of December 31, 2006, Fresenius Medical Care was serving 163,517 patients in 2,108 dialysis clinics.
.gif)
* before one-time expenses, expenses related to the stock option accounting change and the effect of the FTC-related clinic divestitures in the USA; excluding 2005 one-time expenses.
- Excellent sales and earnings growth in all regions
- Integration of Renal Care Group successfully completed
- Outlook 2007: double-digit sales and earnings growth expected
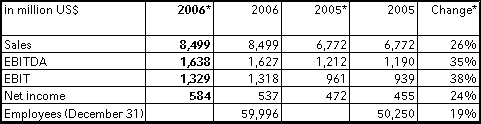
Fresenius Medical Care achieved strong sales growth of 26 % to US$ 8,499 million (2005: US$ 6,772 million). This was mainly driven by the excellent organic growth of 10 % and the consolidation of the Renal Care Group. Sales in dialysis care increased by 31 % to US$ 6,377 million (2005: US$ 4,867 million). In dialysis products Fresenius Medical Care achieved sales of US$ 2,122 million (2005: US$ 1,905 million), an increase of 11 %.
In North America Fresenius Medical Care's sales increased by 32 % to US$ 6,025 million (2005: US$ 4,577 million). Organic growth of 9 % in this region was excellent. Sales outside North America ("International") grew by 13 % (12 % in constant currency) to US$ 2,474 million (2005: US$ 2,195 million).
Net income increased by 18 % to US$ 537 million (2005: US$ 455 million). This result includes one-time expenses of US$ 47 million primarily for the early refinancing of debt, for the integration of Renal Care Group, and expenses related to the stock option accounting change as well as for the after-tax loss on the divestiture of dialysis clinics in the USA. Excluding the above effects and adjusted by one-time expenses in the previous year, net income increased by 24 % to US$ 584 million.
For the full year 2007, Fresenius Medical Care expects revenue to be about US$ 9.4 billion. The net income is expected to be between US$ 675 million and US$ 695 million in 2007.
For further information, please see Fresenius Medical Care's Investor News at www.fmc-ag.com.
Fresenius Kabi
Fresenius Kabi offers infusion therapies and clinical nutrition for seriously and chronically ill patients in the hospital and out-patient environments. The company is also a leading provider of transfusion technology products.

- Strong organic sales growth of 8 %
- EBIT margin at new record levels in the fourth quarter and for the full year 2006
- Outlook 2007: strong margin growth and increase in earnings expected
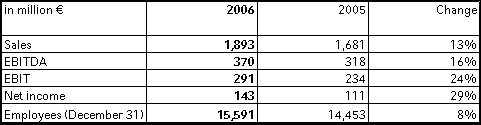
In 2006, Fresenius Kabi's sales increased by 13 % to € 1,893 million (2005: € 1,681 million). The company achieved strong organic growth of 8 %. Acquisitions, primarily Clinico and Australian Pharmatel, contributed 4 % to sales. Currency translation effects contributed 1 % to growth.
Sales in Europe (excluding Germany) increased by 7 %, in Germany by 5 %. Fresenius Kabi continued its exceptional growth outside Europe and achieved sales growth of 41 % in Asia-Pacific, 27 % in Latin America and 17 % in the other regions. Organic growth in the regions outside Europe was again well into the double digits.
Earnings at Fresenius Kabi reached new record levels: EBIT increased by 24 % to € 291 million (2005: € 234 million). The EBIT margin improved to 15.4 % in the full year 2006 (2005: 13.9 %). The original mid-term EBIT margin target of about 15.5 % by 2007 was therefore reached ahead of time. In the fourth quarter, the EBIT margin reached a new record level of 16.0 %. Net profit rose by 29 % to € 143 million (2005: € 111 million). This already includes one-time expenses of € 11 million for the early refinancing of the 2003 Euro Bond.
In 2007, Fresenius Kabi expects a positive sales and earnings performance. Organic sales growth is projected to be 6 to 8 %. Strong sales growth is anticipated again from the regions outside Europe. Based on the positive sales projection and further manufacturing and logistics improvements Fresenius Kabi expects an EBIT margin of 16.0 to 16.5 % in 2007.
Also for the mid-term, Fresenius Kabi expects to continue its positive financial performance: The company targets organic sales growth of 6 to 8 %. Further, Fresenius Kabi foresees its EBIT margin in the 16 to 18 % range.
Fresenius ProServe
Fresenius ProServe is a leading German hospital operator with 55 facilities. Moreover, the company offers engineering and services for hospitals and other health care facilities.

- Very good revenue and earnings development in the hospital operations business
- Strong order intake in engineering and services business
- Outlook 2007: Continued sales and earnings improvements expected
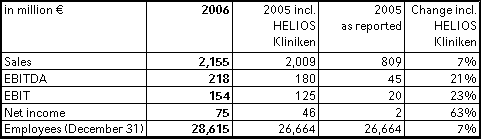
Fresenius ProServe's sales grew by 7 % to € 2,155 million in 2006 (2005 including HELIOS Kliniken: € 2,009 million). Organic growth was 3 %. EBIT increased by 23 % to € 154 million (2005 including HELIOS Kliniken: € 125 million).
Sales in hospital operations (HELIOS Kliniken Group) increased by 8 % to € 1,673 million (2005 including HELIOS Kliniken : € 1,550 million). The sales growth is mainly attributable to the acquisition of HUMAINE Kliniken, which was consolidated as of July 1, 2006. However, HELIOS also achieved strong organic growth of 3 %. EBIT increased to € 133 million, the EBIT margin improved to 7.9 % (2005 including HELIOS Kliniken: € 107 million and 6.9 %).
HELIOS continued its growth strategy in the German hospital market. In January 2007, the company has agreed to acquire two hospitals in North-Rhine Westphalia with a total of 333 beds and sales of about € 32 million in 2006. The acquisition still requires approval by the antitrust authorities.
Sales in the engineering and services business (VAMED, Pharmaplan) increased by 5 % to € 482 million (2005: € 459 million). EBIT rose by 14 % to € 25 million (2005: € 22 million). Order intake and order backlog continued to develop very positively: Order intake increased by 19 % to € 407 million (2005: € 341 million). Order backlog also grew by 19 % to € 428 million as of December 31, 2006 (December 31, 2005: € 360 million).
In December 2006, Fresenius ProServe has signed a definitive agreement to sell its subsidiary Pharmaplan GmbH to NNE A/S (NNE). NNE is a wholly-owned subsidiary of Novo Nordisk A/S, Copenhagen. The approval by the antitrust authorities is expected in the first quarter of 2007. The sale of Pharmaplan is a further step by Fresenius ProServe to focus on its business with hospitals and other healthcare facilities.
Fresenius ProServe projects further improvements in sales and earnings. Future growth potential is mainly expected from hospital privatizations in Germany. For 2007, Fresenius ProServe forecasts organic sales growth of 2 to 3 %. EBIT is expected to increase to € 160 to 170 million.
Video Webcast
As part of the publication of our results for the fiscal year 2006, an analyst conference will be held at the Fresenius headquarters in Bad Homburg on February 22, 2007 at 1:30 p.m. CET (7.30 a.m. EST). All investors are cordially invited to follow the conference in a live broadcast over the Internet at www.fresenius-ag.com / Investor Relations / Presentations. Following the meeting, a recording of the conference will be available as video-on-demand.
Fresenius Group in Figures and Consolidated Statement of Income (US GAAP) see pdf file
Fresenius Medical Care AG & Co. KGaA today announced that it will propose a share split for both classes of shares (ordinary and preferred) in the ratio of 1:3 to the next General Shareholder Meeting on May 15, 2007.
The strong performance of the company in recent years has led to a sharp increase in the share price. The price per ordinary share is currently one of the highest in Germany's DAX index. A share split would significantly lower the price of the individual Fresenius Medical Care share. The proposed share split is intended to promote more trading activity in Fresenius Medical Care shares and to increase the shares' attractiveness for a broader group of investors.
Through a conversion of capital reserves, the subscribed capital of Fresenius Medical Care AG & Co. KGaA is first increased to €295.2 million. The subscribed capital is then divided into 291,449,373 ordinary shares and 3,711,435 preference shares. The new amount of the subscribed capital will then be €1.00 per share. After the share split, every holder of an ordinary share will hold three ordinary shares and every holder of a preference share will hold three preference shares. As a result of the share split, the price level will be reduced arithmetically without affecting the overall value for shareholders.
For American Depositary Share (ADS) Investors:
Fresenius Medical Care shares are traded on the New York Stock Exchange (NYSE) in (NYSE) in the form of ADSs. Currently, the ratio between the ordinary and preference ADS and the underlying ordinary and preference shares is 3:1, meaning that three Fresenius Medical Care ordinary or preference ADSs are the equivalent of one Fresenius Medical Care ordinary or preference share. Upon approval of the proposed share split and the registration with the commercial register, each Fresenius Medical Care ordinary or preference ADS will represent one Fresenius Medical Care ordinary or preference share.
###
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects about 1,500,000 individuals worldwide. Through its network of 2,108 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 163,517 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS-p).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Fresenius today announced encouraging results in the non-ovarian cancer patient stratum of a phase II/III pivotal study on malignant ascites using the trifunctional antibody removab® (catumaxomab). After positive results in the treatment of patients with ascites from ovarian cancer (see Press Release of December 18, 2006 for ovarian stratum), the antibody again showed a clear advantage over a therapy with puncture alone. The median puncture-free survival period (primary endpoint) in the patient group treated with removab® was significantly longer compared to the control group and clinically relevant. The median puncture-free survival was 37 days in the removab® group versus 14 days in the control group (p< 0.0001).
In the subgroup of patients with ascites from gastric cancer (51 % of all non-ovarian cancer patients) the difference was even more marked with a median puncture-free survival of 44 days in the removab® group versus 15 days in the control group (p<0.0001). All other patients (10 % breast, 7 % pancreatic, 6 % colorectal cancer, 26 % others) had a median puncture-free survival of 30 days (removab®) versus 9 days in the control group (p<0.0003). On a pooled basis for both strata (ovarian and non-ovarian cancers) the median puncture-free survival was 46 days in the removab® group versus 11 days in the control group (p<0001).
Positive results were also achieved in key secondary endpoints. Of special importance was the length of time between treatment and first therapeutic puncture (median time to the first therapeutic puncture). In contrast to the primary endpoint, patients who died before the next puncture were not included in this metric. As a result, this secondary endpoint is not affected by the prognosis of these patients at the onset of ascites. The median time to the first therapeutic puncture for all non-ovarian cancers was 80 days (control group: 15 days; p< 0.0001). Patients with gastric cancer benefited especially from the treatment. Median time to the first therapeutic puncture was 118 days in the removab® group versus 15 days in the control group (p< 0.0001). For all other patients the median time to the first therapeutic puncture in the removab® group was 69 versus 15 days in the control group (p< 0.0001). On a pooled basis for both strata (ovarian and non-ovarian cancers) the median time to the first therapeutic puncture was 77 days in the treatment group versus 13 days in the control group (p< 0.0001). In addition, the EpCAM-positive tumor cell concentration in the ascites fluid decreased in patients treated with removab®. At the same time, an increase in CD45-positive leukocytes was seen. Both results indicate a direct anti-tumor effect of the trifunctional antibody.
Removab® showed a very good safety profile in this stratum. This is particularly important as malignant ascites occurs at a very late stage in patients with non-ovarian cancers. Drug-related adverse events due to cytokine release were mild to moderate and mostly fully reversible, with fever, nausea and vomiting being the most common. Pathologic increases of liver parameters and undesirable changes in white blood cell counts were also mild to moderate, transient and mostly without clinical relevance.
"These results continue to support the potential of removab. They demonstrate the significant benefit for ascites patients due to non-ovarian cancers even though they have a worse prognosis compared to ovarian cancer. This indicates that removab® could also play an important role in the treatment of ascites for all underlying non-ovarian cancers," said Dr. Thomas Gottwald, President Fresenius Biotech.
The phase II/III pivotal trial with the trifunctional antibody removab® included a total of 258 patients divided in two strata: ovarian cancer (129 patients) and non-ovarian cancers (129 patients). Based on a 2:1 randomization ratio, the removab® arm in the non-ovarian stratum included 85 patients, of which 62 received all four doses of 10, 20, 50 und 150 µg each. The intraperitoneal infusions were administered over a six-hour period in intervals of three to four days.
Non-ovarian cancer patients account for about 80 % of all malignant ascites cases. The encouraging results of this study significantly increase the number of patients that is potentially eligible for the removab® treatment.
Data on overall survival in connection with the study are expected in the second quarter of 2007 due to the longer follow-up period associated with this secondary endpoint. Market launch of removab® is expected in 2008.
# # #
Puncture-free survival period
Period between the last infusion (control group: day of the puncture) and the first subsequent necessary puncture or death, which ever occurs first.
Trifunctional Antibodies
Trifunctional antibodies are developed by Fresenius Biotech in cooperation with TRION Pharma. Trifunctional antibodies are proteins that bring together cancer cells with two different cell types of the immune system: T-cells and accessory cells (e.g., natural killer cells, macrophages). This mode of action of the trifunctional antibody is the basis for an immune response against the tumor.
# # #
Fresenius Biotech is a company of the Fresenius Group, focused on the development and marketing of biopharmaceuticals in the fields of oncology, immunology and regenerative medicine.
For more information visit the company's website at www.fresenius-biotech.com.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and the ambulatory medical care of patients. In 2006 group sales were about € 10.8 billion. On December 31, 2006 the Fresenius Group had 104,872 employees worldwide.
Fresenius ProServe closed the announced divestiture of its subsidiary Pharmaplan GmbH to NNE A/S on March 31, 2007. All necessary antitrust approvals were received by NNE prior to the completion of the acquisition.
NNE is a wholly-owned subsidiary of Novo Nordisk A/S, Copenhagen, a pharmaceutical company with approximately 23,200 employees worldwide and annual sales of € 5,194 million in 2006.
Pharmaplan provides consulting, engineering and qualification/validation services for the pharmaceutical industry worldwide. The sale of Pharmaplan is a further step by Fresenius ProServe to focus on its two core areas hospital operations (HELIOS Kliniken) and hospital engineering and services (VAMED).
# # #
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and the ambulatory medical care of patients. In 2006 group sales were about € 10.8 billion. On December 31, 2006 the Fresenius Group had 104,872 employees worldwide.
Fresenius Kabi, a European leader in infusion therapy and clinical nutrition, has entered into an agreement with Kyorin Pharmaceutical Co. Ltd., Tokyo, Japan, to acquire its artificial colloid product business. Fresenius Kabi acquires the rights for the production, marketing and sale of these products. Kyorin's artificial colloids sales were € 5.2 million in the fiscal year ended March 31, 2006. The colloids are based on hydroxyethyl starch (HES) and are mainly used in surgical and emergency procedures to substitute blood loss. Kyorin is the only provider of HES products for blood volume replacement in Japan.
In addition, Fresenius Kabi is currently setting up a subsidiary in Tokyo for the marketing and distribution of the acquired products as well as its own product portfolio in Japan. The new subsidiary will allow Fresenius Kabi to enhance its presence in the world's second largest healthcare market.
"In Japan, we are focusing on the intensive care and acute care hospital segments. Kyorin's artificial colloids will perfectly complement Fresenius Kabi's product range. With these products, we will enter the Japanese blood-volume replacement market for the first time. We believe that the high medical benefits and the strong patient safety profile of HES products offer excellent growth opportunities in this attractive market segment", said Mats Henriksson, President of Fresenius Kabi's Asia-Pacific region.
# # #
About Fresenius Kabi
Fresenius Kabi's core product range includes infusion solutions for fluid substitution, blood volume expansion and parenteral nutrition, as well as products for enteral nutrition. Furthermore the company provides concepts for ambulatory health care and is focused on managing and providing home therapies. With its philosophy "Caring for life" and a broad product and service portfolio, the company aims at improving the quality of life of patients all over the world.
The company has 15,591 employees. Fresenius Kabi achieved sales of € 1,893 million and an operating profit of € 291 million in 2006.
Fresenius Kabi AG is a 100% subsidiary of the health care group Fresenius AG.
For details please visit www.fresenius-kabi.com.
About Kyorin
Kyorin is a pharmaceutical company with a long-standing tradition in healthcare. The company is focused on the marketing and sale of drugs for respiratory, otolaryngologic (ear, nose and throat medicine) and urologic diseases.
As of March 31, 2006 (end of the fiscal year), Kyorin Pharmaceutical Co. Ltd. achieved sales of 67.4 bn JPY (approximately € 440 million) and employed 1,502 people worldwide. Kyorin Pharmaceutical Co., Ltd. is a 100% subsidiary of Kyorin Co. Ltd. Kyorin Co. Ltd. is listed on the Tokyo Stock Exchange.
Summary First Quarter 2007
- Net revenue : $ 2,321 million, + 33%
- Operating income (EBIT): $ 365 million, + 50%
- Net income: $ 160 million, + 38%
- Earnings per share: $ 1.63, + 37%
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced its results for the first quarter of 2007.
The operations of Renal Care Group (RCG) are included in the Company's consolidated statements of income and cash flows from April 1, 2006, therefore, the current quarter's results are not directly comparable with the first quarter's results for 2006.
Revenue
Net revenue for the first quarter 2007 compared to the first quarter 2006 increased by 33% to $2,321 million (31% at constant currency). Organic revenue growth worldwide was 9%. Dialysis Services revenue grew by 38% to $1,760 million (37% at constant currency) in the first quarter of 2007. Dialysis Product revenue increased by 18% to $560 million (13% at constant currency) in the same period.
North America revenue increased by 37% to $1,637 million. Dialysis Services revenue increased by 40% to $1,483 million. Average revenue per treatment for the U.S. clinics increased by 6% to $329 in the first quarter 2007 compared to $310 for the same quarter in 2006. Dialysis Product revenue increased by 14% to $153 million led by strong sales of our 2008K hemodialysis machines and the phosphate binding drug PhosLo.
International revenue was $684 million, an increase of 24% (17% at constant currency) compared to the first quarter of 2006. Dialysis Services revenue reached $277 million, an increase of 30% (24% at constant currency). Dialysis Product revenue rose by 20% to $407 million (12% at constant currency), led by strong sales of dialyzers and peritoneal dialysis products.
Earnings
Operating income (EBIT) increased by 50% to $365 million compared to $244 in the first quarter 2006. This translates into an operating margin of 15.7%. For the first quarter 2006, the operating margin was 14.0%.
Compared with the first quarter 2006, the operating margin in North America increased by 200 basis points to 15.8%, due to the consolidation of RCG, revenue rate improvements, the new PhosLo business and higher product sales. In the International segment, the operating margin increased by 30 basis points to 17.6%. The continued strong operational performance in the International segment was driven by increased product sales in all regions.
Net interest expense for the first quarter 2007 was $95 million compared to $56 million in the same quarter of 2006. This increase is entirely attributable to the debt financing for the RCG acquisition.
Income tax expense was $103 million for the first quarter of 2007 compared to $71 million in the first quarter of 2006, reflecting effective tax rates of 38.0% and 37.9%, respectively.
Net income for the first quarter 2007 was $160 million, an increase of 38%. Net income increased by 28% when compared to the first quarter 2006 excluding one-time effects in 2006.
Earnings per share (EPS) for the first quarter of 2007 rose by 37% to $1.63 per ordinary share ($0.54 per American Depositary Share (ADS)) compared to $1.19 ($0.40 per ADS) for the first quarter of 2006. The weighted average number of shares outstanding for the first quarter of 2007 was approximately 98.4 million shares compared to 97.8 million shares for the first quarter of 2006. The increase in shares outstanding results from stock option exercises in 2006 and in the first quarter 2007.
Cash Flow
In the first quarter of 2007, the Company generated $283 million in cash from operations, representing 12% of revenue. The strong cash flow generation was primarily supported by increased earnings.
A total of $109 million was spent for capital expenditures, net of disposals. Free Cash Flow before acquisitions was $174 million compared to $97 million in the first quarter of 2006. A total of $90 million in cash was used for acquisitions. Free Cash Flow after acquisitions was $84 million compared to $87 million last year excluding the acquisition of Renal Care Group.
Please refer to the appendix for a complete overview on the first quarter of 2007.
Patients – Clinics – Treatments
As of March 31, 2007, Fresenius Medical Care treated 169,216 patients worldwide, which represents a 27% increase in patients compared to last year. North America provided dialysis treatments for 118,732 patients, an increase of 32%. Including 32 clinics managed by Fresenius Medical Care North America, the number of patients in North America was 120,603. The International segment served 50,484 patients, an increase of 17% over last year.
As of March 31, 2007, the Company operated a total of 2,194 clinics worldwide. This is comprised of 1,574 clinics in North America, an increase of 35%, and 620 clinics in the International segment, an increase of 16%.
Fresenius Medical Care delivered approximately 6.41 million dialysis treatments worldwide, which represents an increase of 28% year over year. North America accounted for 4.48 million treatments, an increase of 33%, and the International segment delivered 1.93 million treatments, an increase of 17% over last year.
Employees
As of March 31, 2007, Fresenius Medical Care employed 59,076 people (full-time equivalents) worldwide compared to 56,803 employees at the end of 2006. The increase of 2,273 employees is primarily due to the acquisition of Jiate Excelsior Co. Ltd. in Taiwan (announced in January 2007) and continued organic growth in the U.S.
Debt/EBITDA ratio
The ratio of debt to Earnings before Interest, Taxes and Amortization (EBITDA) decreased from 3.81 at the end of first quarter of 2006 to 3.09 at the end of the first quarter 2007. At the end of 2006, the debt/EBITDA ratio was 3.23.
Rating
In the first quarter 2007, Standard & Poor's Ratings Services revised its outlook of the Company to stable from negative. At the same time, the ‘BB' long-term corporate credit ratings on Fresenius Medical Care were affirmed. Moody's upgraded Fresenius Medical Care Senior Secured Credit Facility from Ba2 to Ba1.
Fresenius Medical Care Proposes Share Split of 1:3
On March 21, 2007, the Company announced that it will propose a share split for both classes of shares (ordinary and preference) in the ratio of 1:3 at the next Annual General Meeting being held on May 15, 2007. If approved by the Shareholders, the Company expects the split to become effective in the third quarter of 2007.
Outlook for 2007 Confirmed
For the full year 2007, the Company confirms its outlook and expects to achieve revenue of approximately $9.4 billion. This represents an increase of 11%.
Net income is projected to be in the range of $675 million and $695 million in 2007. This represents an increase of between 18% and 21% on an adjusted basis as compared to 2006 after one-time effects. On a reported basis, this translates into an increase in net income of between 26% and 29%.
In addition, the Company expects spending on capital expenditures and acquisitions to be approximately $650 million in 2007. The debt/EBITDA ratio is projected to be below 3.0 by the end of 2007.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "We are pleased to again deliver exceptionally strong financial results for the first quarter of 2007. Our strong performance was driven by our continuous quality improvement initiatives at the local clinical level, by cost leadership at the regional level, and by our commitment to advance our strategic objectives at the corporate level. The advantages of being the world's only vertically-integrated dialysis provider are increasingly evident as we compete in the global marketplace."
###
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,500,000 individuals worldwide. Through its network of 2,194 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 169,216 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Fresenius Medical Care
Statement of Earnings see PDF-file


