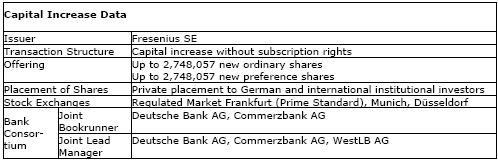
The Management Board of Fresenius SE resolved today, with the consent of the Supervisory Board, to issue up to 2,748,057 new ordinary shares and up to 2,748,057 new preference shares from authorized capital without subscription rights.
The new shares will be placed with institutional investors by way of an accelerated bookbuilt offering. There will be no public offering.
The capital increase is the second component of the long-term financing of the acquisition of APP Pharmaceuticals, Inc. The residual financing requirement will consist of debt instruments.
The acquisition of APP Pharmaceuticals is an important step in the growth strategy of Fresenius Kabi, a business segment of Fresenius SE. With this acquisition, Fresenius Kabi enters the U.S. pharmaceuticals market and achieves a leading position in the global I.V. generics market. This North American platform provides further attractive growth opportunities for Fresenius Kabi's existing product portfolio.
The Else Kröner-Fresenius-Foundation has informed us that, as part of the capital increase, it will purchase approximately 10 % of the new ordinary shares.
After issuance of the new shares, the total number of outstanding ordinary shares and preference shares of Fresenius SE will each increase from currently 77,678,718 to up to 80,426,775.
The new shares are expected to be included in the quotation of the shares of Fresenius SE in the regulated market at the Frankfurt, Munich and Düsseldorf stock exchanges. They will have full dividend entitlement for the fiscal year 2008.
Deutsche Bank and Commerzbank are acting as Joint Lead Managers and Joint Bookrunners and WestLB as Joint Lead Manager for the offering.

About Fresenius SE
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On June 30, 2008 the Fresenius Group had 117,453 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
THIS RELEASE IS FOR INFORMATION PURPOSES ONLY AND MAY NOT BE FURTHER DISTRIBUTED OR PASSED ON TO ANY OTHER PERSON OR PUBLISHED, IN WHOLE OR IN PART, FOR ANY PURPOSE.
This release does not constitute or form part of, and should not be construed as, an offer or invitation to subscribe for, underwrite or otherwise acquire, any securities of Fresenius SE ("Fresenius") or any present or future member of its group nor should it or any part of it form the basis of, or be relied on in connection with, any contract to purchase or subscribe for any securities of Fresenius or any member of its group or any commitment whatsoever. In particular, this release is not an offer of securities in the United States of America (including its territories and possessions), and securities of Fresenius SE may not be offered or sold in the United States of America absent registration under the Securities Act of 1933 (which Fresenius SE does not intend to effect) or pursuant to an exemption from registration.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. This includes the risk that the transaction will not be consummated or on other terms. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
This document is directed at and/or for distribution in the U.K. only to (i) persons who have professional experience in matters relating to investments falling within article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the "Order") or (ii) high net worth entities falling within article 49(2)(a) to (d) of the Order (all such persons being together referred to as "relevant persons"). This document is directed only at relevant persons. Other persons should not act or rely on this document or any of its contents.
The information contained herein is not for publication or distribution in Canada, Australia or Japan and does not constitute an offer of securities for sale in Canada, Australia or Japan.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
As a further component of the acquisition financing of APP Pharmaceuticals, Inc., US$ 2,400 million Senior Secured Credit Facilities were successfully offered in a first phase of syndication. Thereof, US$ 1,900 million will be used for the purchase price, the refinancing of APP's existing debt, transaction fees and expenses.
US$ 500 million will be available for general corporate purposes, including ongoing working capital requirements. The US$ 650 million revolving facilities and the US$ 900 million Term Loan A have a tenor of 5 years, the US$ 850 million Term Loan B of 6 years.
20 of Fresenius' key relationship banks from Europe, North America and Japan, acting as Mandated Lead Arrangers and Joint Lead Arrangers, have provided strong commitments. Therefore, the target amount has been substantially oversubscribed. Following review of customary documentation, the first phase of syndication is expected to close on August 20, 2008. The general syndication to the broad bank market in Europe and the US will be launched in early September.
The total funded debt financing for the APP transaction was already fully underwritten by the three Senior Mandated Lead Arrangers Deutsche Bank (Global Co-ordinator), Credit Suisse and JP Morgan at the time of announcement of the acquisition.
About Fresenius SE
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On June 30, 2008 the Fresenius Group had 117,453 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
ADDITIONAL INFORMATION ABOUT THE MERGER AND WHERE TO FIND IT
In connection with the proposed merger, on August 1, 2008, Fresenius Kabi Pharmaceuticals Holding LLC and APP filed relevant materials with the SEC, including a registration statement that contains a joint prospectus and information statement. Investors and security holders are urged to read these documents and any other relevant documents filed with the SEC, as well as any amendments or supplements to those documents, because they contain important information. Investors and security holders may obtain these documents free of charge at the SEC's website at www.sec.gov. Investors and security holders are urged to read the joint information statement/prospectus and the other relevant materials before making any voting or investment decision with respect to the proposed merger.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius Kabi has successfully closed the acquisition of Dabur Pharma Ltd. In April 2008, the company had announced the acquisition of 73.3 % of the share capital for a price of INR 76.50 per share in cash. Fresenius Kabi has now acquired a further 17.6 % shareholding for a price of INR 76.50 per share in cash through a public offer. Closing of the transaction had also been subject to relevant approvals required under Indian law, which have been received.
The acquisition significantly expands Fresenus Kabi's i.v. drug portfolio and secures its supply of high quality APIs for cytostatics. Dabur Pharma, headquartered in New Delhi, is one of the leading suppliers of generic drugs and active pharmaceutical ingredients (API) to treat cancer. The company holds a substantial number of drug registrations in Asia, Europe and the US.
Dabur Pharma had consolidated revenues of about € 47 million in fiscal year 2007/2008 (April 1, 2007 to March 31, 2008).
Dabur Pharma will be consolidated as from September 1, 2008 in the financial statements of Fresenius Group.
About Fresenius SE
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On June 30, 2008 the Fresenius Group had 117,453 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
About Fresenius Kabi
Fresenius Kabi is the leader in infusion therapy and clinical nutrition in Europe and in its most important countries of Latin America and Asia Pacific. Fresenius Kabi's core product range includes infusion solutions for fluid substitution, blood volume substitution and intravenously administered drugs as well as parenteral and enteral nutrition. Furthermore, the company provides concepts for ambulatory health care and is focused on managing and providing home therapies. With its philosophy "Caring for life" and a comprehensive product portfolio, the company aims at improving the quality of life of patients all over the world. In 2007, Fresenius Kabi achieved sales of € 2,030 million and an operating profit of € 332 million. On June 30, 2008 the company had 18,323 employees.Fresenius Kabi AG is a 100 % subsidiary of the health care group Fresenius SE.
About Dabur
Dabur Pharma Ltd. is committed to the discovery, development and marketing of drugs that fight cancer. Dedicated to its mission of making cancer therapy available to more and more people, it has been expanding ever since inception. The company is the leader in the Indian oncology market and it offers a complete range of products in this segment spanning across injectables, orals, intermediates and APIs and is present in over 40 countries. As of June 30, 2008, the company has 156,677,400 shares outstanding.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
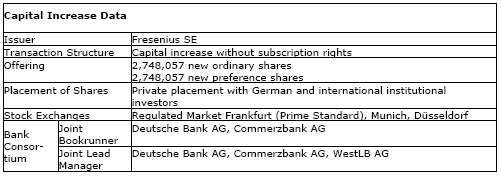
The new Fresenius SE shares were successfully placed today. In connection with the company's capital increase, 2,748,057 new ordinary shares were issued at a price of € 52.00 and 2,748,057 preference shares were issued at a price of € 53.00. The transaction has generated gross proceeds of approx. € 289 million, fully in line with Fresenius' financing plan.
The new shares are expected to be included in the quotation of the existing shares of Fresenius SE in the regulated market at the Frankfurt, Munich and Düsseldorf stock exchanges. They have full dividend entitlement for the fiscal year 2008.
Dr. Ulf Mark Schneider, Chairman of the Management Board of Fresenius SE commented: "The capital increase is a further component of the acquisition financing for APP Pharmaceuticals. This acquisition provides attractive growth opportunities for Fresenius Kabi's existing product portfolio in North America. At the same time, Fresenius Kabi achieves a leading position in the global I.V. generics market. The successful placement of the new shares reflects the confidence of the capital markets in our strategy. With this placement, the entire equity financing of the APP Pharmaceuticals acquisition has been completed within a few weeks."
Deutsche Bank and Commerzbank acted as Joint Lead Managers and Joint Bookrunners and WestLB as Joint Lead Manager for the offering.

About Fresenius SE
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On June 30, 2008 the Fresenius Group had 117,453 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
THIS RELEASE IS FOR INFORMATION PURPOSES ONLY AND MAY NOT BE FURTHER DISTRIBUTED OR PASSED ON TO ANY OTHER PERSON OR PUBLISHED, IN WHOLE OR IN PART, FOR ANY PURPOSE.
This release does not constitute or form part of, and should not be construed as, an offer or invitation to subscribe for, underwrite or otherwise acquire, any securities of Fresenius SE ("Fresenius") or any present or future member of its group nor should it or any part of it form the basis of, or be relied on in connection with, any contract to purchase or subscribe for any securities of Fresenius or any member of its group or any commitment whatsoever. In particular, this release is not an offer of securities in the United States of America (including its territories and possessions), and securities of Fresenius SE may not be offered or sold in the United States of America absent registration under the Securities Act of 1933 (which Fresenius SE does not intend to effect) or pursuant to an exemption from registration.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. This includes the risk that the transaction will not be consummated or on other terms. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
This document is directed at and/or for distribution in the U.K. only to (i) persons who have professional experience in matters relating to investments falling within article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the "Order") or (ii) high net worth entities falling within article 49(2)(a) to (d) of the Order (all such persons being together referred to as "relevant persons"). This document is directed only at relevant persons. Other persons should not act or rely on this document or any of its contents.
The information contained herein is not for publication or distribution in Canada, Australia or Japan and does not constitute an offer of securities for sale in Canada, Australia or Japan.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Moody's today confirmed the Ba1 corporate family rating of Fresenius SE. In July, following Fresenius' announcement of the acquisition of APP Pharmaceuticals, Inc., Moody's had placed the corporate family rating of Fresenius SE under review for possible downgrade.
The rating confirmation reflects Moody's review of the expected capital structure of the acquisition financing, the timeframe of de-leveraging and the benefits of the acquisition on the group's overall business profile. The outlook has been changed from stable to negative.
At the same time, Moody's assigned ratings for the new debt to be issued. The US$ 2.45 billion senior secured credit facility received an investment grade rating of Baa2. The US$ 1.3 billion bridge credit facility was rated at Ba1. These ratings are provisional until the acquisition is closed. The rating of the existing senior unsecured notes remains unchanged.
Stephan Sturm, Chief Financial Officer of Fresenius SE, commented: "Our financing plan for the APP acquisition aimed at minimizing any impact on our credit ratings. With all of the agencies confirming their respective pre-acquisition rating, this target has been fully accomplished."
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On June 30, 2008 the Fresenius Group had 117,453 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
This release contains forward-looking statements that are subject to certain risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to various factors, e.g., changes in the business, economic and competitive environment, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius SE and APP Pharmaceuticals, Inc., have received confirmation that the U.S. Federal Trade Commission (FTC) has completed its review of the proposed acquisition of APP Pharmaceuticals by Fresenius Kabi, a business segment of Fresenius SE. The FTC granted early termination of the waiting period under the Hart-Scott-Rodino Act without conditions. The German antitrust authorities had already approved the acquisition.
On July 7, 2008, Fresenius had announced the acquisition of APP Pharmaceuticals, an important step in the growth strategy of Fresenius Kabi. With the FTC review complete, Fresenius and APP expect the transaction to close mid September 2008, subject to certain other customary closing conditions.
About Fresenius SE
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On June 30, 2008 the Fresenius Group had 117,453 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
About Fresenius Kabi
Fresenius Kabi is the leader in infusion therapy and clinical nutrition in Europe and in its most important countries of Latin America and Asia Pacific. Fresenius Kabi's core product range includes infusion solutions for fluid substitution, blood volume expansion and parenteral nutrition, as well as products for enteral nutrition. Furthermore, the company provides concepts for ambulatory health care and is focused on managing and providing home therapies. With its philosophy "Caring for life" and a comprehensive product portfolio, the company aims at improving the quality of life of patients all over the world. On June 30, 2008 the company had 18,323 employees. In 2007, Fresenius Kabi achieved sales of € 2,030 million and an operating profit of € 332 million. Fresenius Kabi AG is a 100 % subsidiary of the health care group Fresenius SE.
This release contains forward-looking statements that are subject to certain risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to various factors, e.g., changes in the business, economic and competitive environment, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius Kabi, a business segment of Fresenius SE, has completed the acquisition of APP Pharmaceuticals, Inc.
The acquisition is an important step in Fresenius Kabi's growth strategy. Through APP, Fresenius Kabi enters the U.S. pharmaceuticals market and achieves a leading position in the global I.V. generics industry.
Dr. Ulf Mark Schneider, Chairman of the Management Board of Fresenius SE, said: "We are pleased to be able to complete this major transaction in a very short time. Now we are focused on successfully integrating APP and further developing the business. Fresenius and APP share a deep commitment to highest-quality products and medical excellence."
The closing follows completion of the U.S. Federal Trade Commission's (FTC) review of the acquisition. The FTC granted early termination of the waiting period under the Hart-Scott-Rodino Act without conditions. Earlier, German antitrust authorities had also approved the transaction.
Fresenius Kabi had announced the agreement to acquire Schaumburg, Illinois-based APP Pharmaceuticals, Inc., on July 7, 2008.
Fresenius Group expects to consolidate APP Pharmaceuticals in its financial statements as of September 1, 2008.
About Fresenius SE
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On June 30, 2008 the Fresenius Group had 117,453 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
About Fresenius Kabi
Fresenius Kabi is the leader in infusion therapy and clinical nutrition in Europe and in its most important countries of Latin America and Asia Pacific. Fresenius Kabi's core product range includes infusion solutions for fluid substitution, blood volume expansion and parenteral nutrition, as well as products for enteral nutrition. Furthermore, the company provides concepts for ambulatory health care and is focused on managing and providing home therapies. With its philosophy "Caring for life" and a comprehensive product portfolio, the company aims at improving the quality of life of patients all over the world. On June 30, 2008 the company had 18,323 employees. In 2007, Fresenius Kabi achieved sales of € 2,030 million and an operating profit of € 332 million. Fresenius Kabi AG is a 100 % subsidiary of the health care group Fresenius SE.
This release contains forward-looking statements that are subject to certain risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to various factors, e.g., changes in the business, economic and competitive environment, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
ADDITIONAL INFORMATION ABOUT THE MERGER AND WHERE TO FIND IT
In connection with the proposed merger, Fresenius Kabi Pharmaceuticals Holding, Inc. and APP have filed relevant materials with the SEC, including a registration statement that contains a joint prospectus and information statement. Investors and security holders are urged to read these documents and any other relevant documents filed with the SEC, as well as any amendments or supplements to those documents, because they contain important information. Investors and security holders may obtain these documents free of charge at the SEC's website at www.sec.gov. Investors and security holders are urged to read the joint information statement/prospectus and the other relevant materials before making any investment decision with respect to the proposed merger.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Fresenius Medical Care AG & Co. KGaA, the world's largest provider of dialysis products and services, today announced the closing of an exclusive license agreement with Luitpold Pharmaceuticals, Inc., and its subsidiary American Regent, Inc. for the manufacture and distribution of intravenous (I.V.) iron products in the USA.
The closing follows completion of the US Federal Trade Commission's (FTC) review of the license agreement under the Hart-Scott-Rodino Act and the issuance of a consent order to permit the closing.
In July 2008, Fresenius Medical Care entered into a separate and independent license and distribution agreement with Galenica Ltd. and its subsidiary Vifor Pharma, for certain countries in Europe and the Middle East, to market and distribute intravenous Iron products, such as Venofer® and Ferinject® for dialysis treatment.
In the U.S., the license agreement among Fresenius USA Manufacturing, Inc. (FUSA), Luitpold Pharmaceuticals Inc., and American Regent, Inc. provides FUSA with exclusive rights to manufacture and distribute Venofer® to freestanding (non-hospital based) US dialysis facilities. Luitpold Pharmaceuticals will continue to sell Venofer® for use in treating chronic kidney disease patients not yet on dialysis and in treating patients with renal failure in hospitals.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "We are pleased to be able to complete this agreement which strengthens our ability to improve the treatment of Iron Deficiency Anemia experienced by dialysis patients. We are delighted to have Galenica Ltd., Vifor Pharma, Luitpold Pharmaceuticals, Inc. and American Regent, Inc., as partners. The license to make and distribute these products is an important milestone in the execution of Fresenius Medical Care's renal pharma strategy."
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,318 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to more than 180,000 patients around the globe (as of June 30, 2008). Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P). For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
Galenica is a diversified group active throughout the healthcare market which, among other things, develops, manufactures and commercialises pharmaceutical products, runs pharmacies, provides logistical and database services and sets up networks. The Galenica Group enjoys a leading position in all its business sectors – Pharma, Logistics, HealthCare Information and Retail. A large part of the Group's income is generated by international operations. Additional information on the Galenica Group can be found atwww.galenica.com.
Luitpold Pharmaceuticals, Inc., headquartered in Shirley, NY, manufactures and distributes over 65 pharmaceutical products including Venofer® (iron sucrose injection, USP), the leading IV iron therapy in the U.S., through its human health subsidiary, American Regent, Inc. Luitpold Pharmaceuticals, Inc., a Daiichi-Sankyo Group company, also markets dental bone regeneration products and veterinary pharmaceuticals through its Osteohealth and Animal Health divisions. Daiichi Sankyo Company, Ltd., established in 2005 after the merger of two leading century-old Japanese pharmaceutical com-panies, is a global pharmaceutical innovator, continuously generating innovative drugs that enrich the quality of life for patients around the world. For more information about Luitpold see www.luitpold.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
After successfully raising more than US$ 1,300 million from the issuance of mandatory exchangeable bonds and new shares in July and August, Fresenius has met strong reception in the general syndication phase of its Senior Secured Credit Facilities used to finance the acquisition of APP Pharmaceuticals, Inc.
Given strong demand from institutional investors, resulting in substantial oversubscription, Fresenius has increased the Facilities by US$ 500 million to US$ 2,950 million.
Prior to general syndication, the Facilities consisted of revolving credit facilities of US$ 450 million with a maturity of 5 years, a US$ 1,000 million Term Loan A with a maturity of 5 years and a US$ 1,000 million Term Loan B with a maturity of 6 years. The Term Loan B has now been increased to US$ 1,500 million.
Standard & Poor's today announced that the ratings of Fresenius SE and its various debt instruments remain unchanged following the US$ 500 million increase of the Facilities.
The additional funds will be used to reduce the US$ 1,300 million bridge credit facility which was drawn at the closing of the APP Pharmaceuticals acquisition in early September, to US$ 800 million. The bridge credit facility has a maturity of up to 7 years.
Fresenius is a health care group with international operations, providing products and services for dialysis, hospital and outpatient medical care. In 2007, group sales were approx. € 11.4 billion. On June 30, 2008 the Fresenius Group had 117,453 employees worldwide.
For more information visit the Company's website at www.fresenius.com.
This release contains forward-looking statements that are subject to certain risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to various factors, e.g., changes in the business, economic and competitive environment, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Board of Management: Dr. Ulf M. Schneider (President and CEO), Rainer Baule, Dr. Francesco De Meo, Dr. Jürgen Götz, Dr. Ben Lipps, Stephan Sturm, Dr. Ernst Wastler
Supervisory Board: Dr. Gerd Krick (Chairman)
Registered Office: Bad Homburg, Germany/Commercial Register No. HRB 10660
Summary Third Quarter 2008:
|
Net revenue |
$ 2,713 million |
|
+ 12% |
|
Operating income (EBIT) |
$ 422 million |
|
+ 6% |
|
Net income |
$ 206 million |
|
+ 14% |
|
Earnings per share |
$ 0.69 |
|
+ 14% |
Summary First Nine Months 2008:
|
Net revenue |
$ 7,890 million |
|
+ 10% |
|
Operating income (EBIT) |
$ 1,240 million |
|
+ 8% |
|
Net income |
$ 603 million |
|
+ 16% |
|
Earnings per share |
$ 2.03 |
|
+ 16% |
Fresenius Medical Care AG & Co. KGaA ("the Company"), the world's largest provider of Dialysis Products and Services, today announced its results for the third quarter and first nine months of 2008.
Third Quarter 2008:
Revenue
Net revenue for the third quarter of 2008 increased by 12% to $2,713 million (9% at constant currency) compared to the third quarter of 2007. Organic revenue growth worldwide was 8%. Dialysis Services revenue grew by 10% to $1,985 million (9% at constant currency) in the third quarter of 2008. Dialysis Product revenue increased by 16% to $728 million (11% at constant currency) in the same period.
North America revenue increased by 7% to $1,771 million. Organic revenue growth was 5%. Dialysis Services revenue grew by 6% to $1,587 million. Average revenue per treatment for the U.S. clinics increased to $333 in the third quarter of 2008. This represents an increase of $6 per treatment compared to the third quarter of 2007 as well as sequentially from the second quarter of 2008. The improvement in the revenue per treatment was primarily due to increased commercial revenue rates. Dialysis Product revenue increased by 11% to $184 million. This performance was led by strong sales across almost the entire product portfolio.
International revenue was $942 million, an increase of 23% (14% at constant currency) compared to the third quarter of 2007. Organic revenue growth in the International segment was 13%. Dialysis Services revenue reached $398 million, an increase of 30% (20% at constant currency). Dialysis Product revenue rose 19% to $544 million (11% at constant currency), led by strong dialyzer and dialysis machine sales.
Earnings
Operating income (EBIT) increased by 6% to $422 million compared to $397 million in the third quarter of 2007, resulting in an operating margin of 15.6% compared to 16.4% for the third quarter 2007. This margin decrease mainly reflected higher personnel expenses, increased costs for the anticoagulant drug Heparin, a mix effect with accelerated growth in the International Service business, start-up costs of new clinics and higher expenditures for our research and development activities. Further, we experienced higher depreciation expenses as a result of our recent investments to expand our production capacities to continue to meet customer demand. The availability of these new capacities allowed a more normalized summer shutdown program for maintenance of our European facilities, in contrast to last year's shortened program. The exceptional revenue growth was supported by increased reimbursement rates and a continued above market growth of renal products.
Net interest expense for the third quarter of 2008 was $87 million compared to $95 million in the same quarter of 2007. This positive development was mainly attributable to lower average interest rates associated with changes in the financing structure due to the redemption of a portion of the Trust Preferred Securities.
Income tax expense was $123 million for the third quarter of 2008 compared to $115 million in the third quarter of 2007, reflecting effective tax rates of 36.6% and 38.0%, respectively. The decrease is mainly a result of German tax reform which became effective January 1, 2008.
Net income for the third quarter 2008 was $206 million, an increase of 14%.
Earnings per share (EPS) for the third quarter of 2008 rose 14% to $0.69 per ordinary share compared to $0.61 for the third quarter of 2007. Earnings per ordinary American Depository Share (ADS) are equivalent as one ADS represents one share as a result of the change in ratio of the Company's ordinary shares and preference shares to ADSs. The weighted average number of shares outstanding for the third quarter of 2008 was approximately 297.2 million shares compared to 295.8 million shares for the third quarter of 2007. The increase in shares outstanding is due to stock option exercises in the fourth quarter of 2007 and in the first nine months of 2008.
Cash Flow
In the third quarter of 2008, the Company generated a very strong $315 million in cash from operations, representing 12% of revenue. The cash flow generation was impacted by our strong operating income combined with a slight increase in working capital.
A total of $160 million was spent for capital expenditures, net of disposals. Free Cash Flow before acquisitions was $155 million. A total of $39 million in cash was used for acquisitions, net of divestitures.
Nine Months Ended September 30, 2008:
Revenue and Earnings
Net revenue was $7,890 million, up 10% from the first nine months of 2007. In constant currency net revenue rose 7%. Organic growth was 7% in the first nine months of 2008.
Operating income (EBIT) increased by 8% to $1,240 million compared to $1,152 million in the first nine months of 2007, resulting in an operating margin of 15.7% compared to 16.1% for the first nine months of 2007. This development mainly reflected higher research and development expenses and start-up costs for new clinics. Reduced reimbursement rates for EPO, lower utilization levels of EPO as well as increased costs for the anticoagulant drug Heparin and higher personnel expenses were partially offset by increases in underlying reimbursement rates and strong contributions from renal products.
Net interest expense for the first nine months of 2008 was $252 million compared to $281 million in the same period of 2007. The reduction was mainly due to lower average interest rates associated with changes in our financing structure.
Income tax expense was $366 million in the first nine months of 2008 compared to $331 million in the same period in 2007, reflecting tax rates of 37.0% and 38.0%, respectively.
For the first nine months of 2008, net income was $603 million, an increase of 16% from the first nine months of 2007.
Earnings per ordinary share rose 16% to $2.03. The weighted average number of shares outstanding during the first nine months of 2008 was approximately 296.8 million.
Cash Flow
Cash from operations during the first nine months of 2008 was $716 million, representing 9% of revenue. Cash Flow generation was impacted by our strong operating income, partially offset by slight increases in the Days Sales Outstanding (DSO) and other working capital.
A total of $493 million was used for capital expenditures, net of disposals. Free Cash Flow before acquisitions for the first nine months of 2008 was $223 million. A total of $130 million in cash was used for acquisitions, net of divestitures.
Please refer to the attachments for a complete overview on the third quarter and first nine months of 2008.
Patients – Clinics – Treatments
As of September 30, 2008, Fresenius Medical Care treated 181,937 patients worldwide, which represents a 6% increase compared to last year. North America provided dialysis treatments for 125,356 patients, an increase of 4%. Including 34 clinics managed by Fresenius Medical Care North America, the number of patients in North America was 127,172. The International segment served 56,581 patients, an increase of 10% over last year.
As of September 30, 2008, the Company operated a total of 2,349 clinics worldwide. This is comprised of 1,666 clinics in North America (1,700 including managed clinics), an increase of 5%, and 683 clinics in the International segment, an increase of 8%.
Fresenius Medical Care delivered approximately 20.7 million dialysis treatments worldwide during the first nine months of 2008. This represents an increase of 5% year over year. North America accounted for 14.2 million treatments, an increase of 4%, and the International segment delivered 6.4 million treatments, an increase of 9% over last year.
Employees
As of September 30, 2008, Fresenius Medical Care had 63,990 employees (full-time equivalents) worldwide compared to 61,406 employees at the end of 2007.
Debt/EBITDA Ratio
The ratio of debt to Earnings before Interest, Taxes and Amortization (EBITDA) decreased from 2.88 at the end of the third quarter of 2007 to 2.71 at the end of the third quarter 2008.
Credit Ratings
During Q3, Moody´s did not change any Rating of Fresenius Medical Care. Standard & Poors revised its outlook on July 9th, 2008 from positive to negative in connection with Fresenius SE´s acquisition of APP Pharmaceuticals Inc. All other Ratings of Fresenius Medical Care were affirmed, last on September 4th, 2008. For further detailed information on Fresenius Medical Care´s Credit Relations we would like to refer you to our Internet Page at www.fmc-ag.com / InvestorRelations / CreditRelations where one can find for example additional information on our credit ratings, maturity profiles and credit instruments.
Outlook for 2008
For the year 2008, the Company confirms its outlook and expects to achieve revenue of more than $10.4 billion, an increase of more than 7%.
Net income is projected to be between $805 million and $825 million in the fiscal year 2008. This represents an increase of 12% to 15%.
In addition, the Company expects to spend $650 to $750 million on capital expenditures and $150 to $250 million on acquisitions. The debt/EBITDA ratio is projected to decrease to below 2.8 by the end of 2008.
For 2010, Fresenius Medical Care continues to expect revenue of more than $11.5 billion. Earnings after tax are projected to grow in the low- to mid-teens each year.
Ben Lipps, Chief Executive Officer of Fresenius Medical Care, commented: "We are very pleased to report a strong third quarter and first nine months of 2008. Our organic revenue growth clearly accelerated during the year 2008 showing an excellent growth of 8% in the third quarter of 2008. With 12% of revenue, we have seen a very strong operating cash flow performance. We continued our investments in future growth by expanding our clinic network and production capacities as well as our research and development activities. Despite cost pressures, an uncertain economic environment and volatile currency developments, we are proud to say that we can reconfirm our guidance for 2008 and are confident of achieving our mid term financial targets for 2010. More importantly in the current environment, our financing is very stable through 2011. We remain focused on continuing to execute our strategic objectives, in particular, providing our patients the best dialysis treatment possible to ensure a maximum of quality of life."
Conference Call
Fresenius Medical Care will hold a conference call to discuss the results of the third quarter and the first nine months of 2008 on Tuesday, November 4, 2008, at 3:30 pm CEDT / 9:30 am EDT. The Company invites journalists to view the live webcast of the conference call at the Company's website at www.fmc-ag.com / Investor Relations / Presentations. A replay will be available shortly after the call.
Fresenius Medical Care is the world's largest integrated provider of products and services for individuals undergoing dialysis because of chronic kidney failure, a condition that affects more than 1,600,000 individuals worldwide. Through its network of 2,318 dialysis clinics in North America, Europe, Latin America, Asia-Pacific and Africa, Fresenius Medical Care provides dialysis treatment to 179,340 patients around the globe. Fresenius Medical Care is also the world's leading provider of dialysis products such as hemodialysis machines, dialyzers and related disposable products. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME, FME3) and the New York Stock Exchange (FMS, FMS/P).
For more information about Fresenius Medical Care visit the Company's website at www.fmc-ag.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care
Statement of Earnings
see PDF-file


