- The European Investment Bank (EIB) loan of €400 million will support Fresenius investments to increase availability of innovative medicines and biosimilars across Europe.
- The loan demonstrates EIB’s ongoing commitment to high-quality and affordable healthcare.
- This financing is a further step in the #FutureFresenius agenda.
Fresenius, a global healthcare company, will receive a new €400 million loan from the European Investment Bank (EIB) to strengthen Fresenius’ European research and development (R&D) activities. The financing will be used to support expansion of Fresenius Kabi's manufacturing of medical products and biosimilars in European countries.
The EIB-backed investments aim to strengthen resilience of pharmaceutical production in the EU and contribute to security of supply and financial relief for European healthcare systems. This will facilitate access to modern and affordable healthcare.
Sara Hennicken, CFO of Fresenius: "Our mission at Fresenius is to save and improve people’s lives. Investing in our core business with the development of innovative, affordable healthcare products is a key element of Rejuvenate, the current phase of our #FutureFresenius journey. Consequently, the continued trust of the European Investment Bank means more to us than just attractive financing; it is a valued recognition of our contribution to a healthy European future."
Nicola Beer, Vice President of the EIB: "Our long-standing partnership with Fresenius is a testament to the EIB’s enduring commitment to accessible, high-quality healthcare throughout Europe. By supporting the accelerated development of biosimilar and generic pharmaceuticals by Fresenius across several European countries, we are helping to deliver innovative, affordable solutions for millions of patients while strengthening the EU’s resilience in medicine supply and research excellence. Together, we are scaling up scientific advances and manufacturing capability, paving the way for a healthier, more sustainable future."
According to a study by IQVIA, generic drugs account for around 70% of prescriptions in Europe but represent only just under 20% of pharmaceutical costs, according to an IQVIA research.
As a manufacturer of pharmaceuticals, biosimilars, clinical nutrition and medical technologies with around 20 manufacturing plants and multiple R&D centers across Europe, Fresenius stands for the reliable supply of essential medicines.
Over the last five years, Fresenius has invested more than €1 billion in European manufacturing in key markets. These investments are intended to meet European demand. The aim of Fresenius' local-for-local strategy is to manufacture products for European patients in Europe.
Over the last 5 years, the EIB has provided more than €22 billion in financing for the health and life sciences sector, including €4 billion in countries outside the EU.
The EIB has backed long term innovation investments by Fresenius for around 20 years.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
- The European Investment Bank (EIB) loan of €400 million will support Fresenius investments to increase availability of innovative medicines and biosimilars across Europe.
- The loan demonstrates EIB’s ongoing commitment to high-quality and affordable healthcare.
- This financing is a further step in the #FutureFresenius agenda.
Fresenius, a global healthcare company, will receive a new €400 million loan from the European Investment Bank (EIB) to strengthen Fresenius’ European research and development (R&D) activities. The financing will be used to support expansion of Fresenius Kabi's manufacturing of medical products and biosimilars in European countries.
The EIB-backed investments aim to strengthen resilience of pharmaceutical production in the EU and contribute to security of supply and financial relief for European healthcare systems. This will facilitate access to modern and affordable healthcare.
Sara Hennicken, CFO of Fresenius: "Our mission at Fresenius is to save and improve people’s lives. Investing in our core business with the development of innovative, affordable healthcare products is a key element of Rejuvenate, the current phase of our #FutureFresenius journey. Consequently, the continued trust of the European Investment Bank means more to us than just attractive financing; it is a valued recognition of our contribution to a healthy European future."
Nicola Beer, Vice President of the EIB: “Our long-standing partnership with Fresenius is a testament to the EIB’s enduring commitment to accessible, high-quality healthcare throughout Europe. By supporting the accelerated development of biosimilar and generic pharmaceuticals by Fresenius across several European countries, we are helping to deliver innovative, affordable solutions for millions of patients while strengthening the EU’s resilience in medicine supply and research excellence. Together, we are scaling up scientific advances and manufacturing capability, paving the way for a healthier, more sustainable future.”
Generic drugs account for around 70% of prescriptions in Europe but represent only just under 20% of pharmaceutical costs, according to an IQVIA research.
As a manufacturer of pharmaceuticals, biosimilars, clinical nutrition and medical technologies with around 20 manufacturing plants and multiple R&D centers across Europe, Fresenius stands for the reliable supply of essential medicines.
Over the last 5 years, Fresenius has invested more than €1 billion in European manufacturing in key markets. These investments are intended to meet European demand. The aim of Fresenius' local-for-local strategy is to manufacture products for European patients in Europe.
Over the last 5 years, the EIB has provided more than €22 billion in financing for the health and life sciences sector, including €4 billion in countries outside the EU.
The EIB has backed long term innovation investments by Fresenius for around 20 years.
About the EIB
The European Investment Bank (ElB) is the long-term lending institution of the European Union, owned by its Member States. Built around eight core priorities, we finance investments that contribute to EU policy objectives by bolstering climate action and the environment, digitalisation and technological innovation, security and defence, cohesion, agriculture and bioeconomy, social infrastructure, the capital markets union, and a stronger Europe in a more peaceful and prosperous world.
The EIB Group, which also includes the European Investment Fund (EIF), signed nearly €89 billion in new financing for over 900 high-impact projects in 2024, boosting Europe’s competitiveness and security.
All projects financed by the EIB Group are in line with the Paris Climate Agreement, as pledged in our Climate Bank Roadmap. Almost 60% of the EIB Group’s annual financing supports projects directly contributing to climate change mitigation, adaptation, and a healthier environment.
Fostering market integration and mobilising investment, the Group supported a record of over €100 billion in new investment for Europe’s energy security in 2024 and mobilised €110 billion in growth capital for startups, scale-ups and European pioneers. Approximately half of the EIB's financing within the European Union is directed towards cohesion regions, where per capita income is lower than the EU average.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Else Kröner Award 2026
You can find all information regarding the Else Kröner Award 2026 in your local intranet.
This page provides the Participation Guidelines as well as the Data Privacy Information.
In case of questions please contact the Else Kröner Award Team via elsekroeneraward@fresenius.com.
Fresenius continues to reduce complexity and increase efficiency in its global network. In line with #FutureFresenius, the company announces the divestment of Fresenius Kabi’s Calea homecare business in Canada. This includes the divestment of four sites across the country, including the head office in Mississauga, Ontario, as well as the offices in Hamilton, Ontario, Burnaby, British Columbia, and Calgary, Alberta.
The company has sold the Calea business in Canada to Capital Health Partners (CHP), a Canadian-owned and operated healthcare company committed to advancing the delivery of medical supplies, equipment, pharmaceuticals, and pharmacy services across Canada. Fresenius Kabi continues to supply the Canadian market with its broad portfolio of products and thereby remains a close partner to health care delivery in Canada.
Fresenius continues to reduce complexity and increase efficiency in its global network. In line with #FutureFresenius, the company announces the divestment of Fresenius Kabi’s Calea homecare business in Canada. This includes the divestment of four sites across the country, including the head office in Mississauga, Ontario, as well as the offices in Hamilton, Ontario, Burnaby, British Columbia, and Calgary, Alberta.
The company has sold the Calea business in Canada to Capital Health Partners (CHP), a Canadian-owned and operated healthcare company committed to advancing the delivery of medical supplies, equipment, pharmaceuticals, and pharmacy services across Canada. Fresenius Kabi continues to supply the Canadian market with its broad portfolio of products and thereby remains a close partner to health care delivery in Canada.
The global healthcare company Fresenius is collaborating with other companies and academic institutions with the goals of accelerating the manufacturing of CAR-T cell therapy, making it more cost-effective, and improving patient access across Europe. Led by Fresenius, the newly launched EASYGEN (Easy workflow integration for gene therapy) consortium will focus on efforts to develop a modular, hospital-based platform capable of manufacturing personalized cell therapies in just a few days, rather than weeks. The project is a public-private partnership, with €8 million in funding provided by the EU through the Innovative Health Initiative (IHI). It leverages technology originally developed by the Cell and Gene Therapy team of Fresenius Kabi, part of Fresenius.
Read the full press release here.
Physicians, researchers, and partner institutions across Europe aim to deliver innovative, personalized therapies more quickly
Development project focused on a hospital-based modular platform, based on technology initially developed by Fresenius Kabi
The EASYGEN project is a public-private partnership, with €8 million backed by EU funding through the Innovative Health Initiative
Important step in #FutureFresenius program
The global healthcare company Fresenius is collaborating with other companies and academic institutions with the goals of accelerating the manufacturing of CAR-T cell therapy, making it more cost-effective, and improving patient access across Europe. Led by Fresenius, the newly launched EASYGEN (Easy workflow integration for gene therapy) consortium will focus on efforts to develop a modular, hospital-based platform capable of manufacturing personalized cell therapies in just a few days, rather than weeks. The project is a public-private partnership, with €8 million in funding provided by the EU through the Innovative Health Initiative (IHI). It leverages technology originally developed by the Cell and Gene Therapy team of Fresenius Kabi, part of Fresenius.
Dr. Christian Hauer, President MedTech at Fresenius Kabi, said: “This project contributes to expanding our MedTech platform, making it an important step on our path to #FutureFresenius. The aim is not only to develop cutting-edge medical technologies, but also to make them available quickly, safely, and close to the patient. In this way, we are actively working to shape the healthcare of tomorrow.”
“EASYGEN brings together physicians, researchers, and partner institutions from across Europe with the goal of collaboratively advancing innovative, personalized therapies such as CAR-T cells for cancer treatment. Automation can help reduce production complexity of these therapies, with the aim of making it easier to scale these life-saving treatments and improve patient access,” added Prof. Dr. med. Ralf Kuhlen, Chief Medical Officer at Fresenius.
CAR-T therapy is a breakthrough treatment that involves genetically modifying a patient’s T cells to target cancer. It requires complex, time-intensive production in specialized facilities often far from patients. Limited manufacturing capacity and supply chain delays can potentially prevent timely patient access. Despite clinical eligibility, access to CAR-T cell therapy remains limited for patients across Europe. This is particularly evident in diffuse large B-cell lymphoma (LBCL), a type of cancer that is one of the most common indications: Across five European countries, the average treatment rate is below 20%. While approximately 30% of eligible patients receive CAR-T therapy in France, the figure drops to just 11% in Italy.1
Fresenius is actively involved in cell and gene therapy. Fresenius Kabi provides medical technology for these therapies, including automated cell processing systems such as Lovo and Cue. Fresenius Helios, for example, at its Helios Hospital Berlin-Buch, has been offering CAR-T cell therapy as a standard treatment for relapsed cases since 2019. The clinic is also conducting clinical trials to further explore the potential of CAR-T therapies. Quirónsalud, Fresenius’ Spanish hospital business, has established specialized oncology units that offer CAR-T cell therapy as part of their advanced cancer treatment portfolio, particularly for hematologic malignancies.
EASYGEN is led by Fresenius and academically co-led by Fraunhofer Institute, IZI, Leipzig – one of Europe’s foremost immunotherapy research centers in collaboration with Prof. Dr. Michael Hudecek, a leader in CAR-T cell engineering and Prof. Dr. Ulrike Köhl, a pioneer in translational cellular immunotherapies.
1 IQVIA Institute for Human Data Science. (03/ 2025). Achieving CAR T-cell Therapy Health System Readiness: An Assessment of Barriers and Opportunities.
Consortium partners – 18 organizations across 8 countries
Industry & clinical leaders: Fresenius (Coordinator, Germany), Helios Hospital Berlin-Buch (Germany), Quirónsalud (Spain), Fenwal Inc. (USA), Cellix Ltd. (Ireland), Charles River (Germany), Pro-Liance Global Solutions (Germany), TQ Therapeutics (Germany), Philips Electronics Nederland B.V. (Netherlands).
Academic & research institutions: Fraunhofer IESE (Germany), Fraunhofer IZI (Germany), Helmholtz-Zentrum Dresden-Rossendorf (Germany), Technical University of Denmark (Denmark), Frankfurt School of Finance & Management (Germany), European Society for Blood & Marrow Transplantation (Netherlands), Bar-Ilan University (Israel), University of Glasgow (UK), University of Navarra (Spain).
* * *
Learn more about CAR-T cell therapy: Interview Prof. Bertram Glaß, Chief Physician for Hematology and Cell Therapy at Helios Hospital Berlin-Buch: www.fresenius.com/car-t-cell-therapy
* * *
About EASYGEN
EASYGEN is a five-year research project supported by the Innovative Health Initiative Joint Undertaking (IHI JU) under grant agreement No 101194710. The JU receives support from the European Union’s Horizon Europe research and innovation programme and COCIR, EFPIA, Europa Bío, MedTech Europe, Vaccines Europe and industry partners. Selected under the IHI call “User-centric technologies and optimized hospital workflows for a sustainable healthcare workforce”, the project aims to develop an integrated, automated platform that enables point-of-care CAR-T cell manufacturing—cutting production time, reducing costs, and expanding access to next-generation immunotherapies.
Disclaimer: Funded by the European Union, the private members, and those contributing partners of the IHI JU. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the aforementioned parties. Neither of the aforementioned parties can be held responsible for them.

Copyright: Johannes Krzeslack
Image Description
In the front row, from left to right: Dr. Sonja Steppan (Easygen Principal Investigator, Fresenius SE), Prof. Dr. Michael Hudecek (Fraunhofer IZI), Theresa Kagerbauer (TQ Therapeutics), Dr. Agnes Vosen (HZDR), Christopher Wegener (Fresenius Kabi), Vaclovas Radvilas (EBMT), Dr. Julia Schüler (Charles River), Dr. Julia Busch-Casler (HZDR), Nicole Spanier-Baro (Fraunhofer IESE), Vivienne Williams (Cellix Limited), Prof. Dr. Bertram Glaß (Helios), Prof. Dr. Ulrike Köhl (Fraunhofer IZI), Rebecca Scheiwe (Fresenius SE). In the back row, from left to right: Prof. Dr. Ralf Kuhlen (Fresenius SE), Prof. Dr. Jens O. Brunner (DTU), Dominik Narres (Fresenius SE), Thomas Brzoska (Pro-Liance Global Solutions), Dr. David Krones (Fraunhofer IZI), Dr. Sabine Bertsch (Pro-Liance Global Solutions), Dr. Ralf Hoffmann (Philips), Christin Zündorf (TQ Therapeutics), Dr. Anna Dünkel (Fraunhofer IZI).

This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius SE & Co. KGaA
Registered Office: Bad Homburg, Germany / Commercial Register: Amtsgericht Bad Homburg, HRB 11852
Chairman of the Supervisory Board: Wolfgang Kirsch
General Partner: Fresenius Management SE
Registered Office: Bad Homburg, Germany / Commercial Register: Amtsgericht Bad Homburg, HRB 11673
Management Board: Michael Sen (Chairman), Pierluigi Antonelli, Sara Hennicken, Robert Möller, Dr. Michael Moser
Chairman of the Supervisory Board: Wolfgang Kirsch
Quality excellence and integrity
In everything we do, we have a clear compass: it is all about doing the right thing.
As a global healthcare Group, our goal is to save lives and improve the quality of life for our patients. To achieve this, we provide access to affordable, innovative medical products and high-quality clinical care, and create a framework for the safe handling of medicines.
Integrity is the cornerstone of trust − within our organization and in every interaction with patients, partners, regulators, and society at large. At Fresenius, we foster a culture where ethical behavior is embedded in everything we do. Guided by clear principles, we don’t simply comply with standards − we act with conviction. Our commitment to respect human rights is an integral part of our social responsibility.
The Ethical Foundation of our Sustainability Framework encompasses the following focus topics:
For us, quality excellence means a commitment to providing leading product and service quality and safety, driven by continuous improvement and effectiveness. We foster innovation that delivers meaningful benefits for patients.
Since our operations encompass healthcare services, pharmaceuticals, and medical technology, the quality requirements naturally vary. Our quality management systems are specifically designed to address these diverse needs, meeting both internal standards and external requirements. We ensure this through robust processes and comprehensive training programs across all areas of our business. To track our performance, we use clearly defined quality indicators and targets. These are not only embedded in our daily work but also tied to the variable compensation of our Management Board.
➔ Read more in our Sustainability Statement.

Innovation is key to meeting the needs of our patients – today and in the future. For us, it is a driving force behind improving treatment options, streamlining workflows, and delivering high-quality healthcare solutions. Our integrated approach spans the entire value chain and focuses, among other things, on initiatives that enhance therapies and patient experience through research, telemedicine, and Artificial Intelligence.

The quality of our products, services, and therapies is the basis for optimal medical care.
➔ Read more in our Sustainability Statement.
We are committed to respecting human rights.
Patients, doctors, nursing and administrative staff rely on our products, concepts, and solutions. Around 180,000 employees place their trust in Fresenius as an employer. At the same time, we rely on thousands of people worldwide who work for our suppliers and business partners in our value chain.
Our Human Rights Program is based on internationally recognized standards and frameworks, including the Universal Declaration of Human Rights, the Core Labor Standards of the International Labour Organization, the UN Guiding Principles on Business and Human Rights, and the OECD Due Diligence Guidance for Responsible Business Conduct.

Our commitment to respect human rights is an integral part of our responsibility as a global healthcare company.
➔ Read more on our Human Rights page.
We are committed to high ethical business standards for the benefit of patients, employees, and the planet.
We believe in doing the right thing. That means acting not only in line with legal requirements, but also in accordance with industry codes, internal policies, and our values – the Fresenius Principles. Internal and external controls help ensure compliance and safeguard the trust our stakeholders place in us.

No matter the role, our work is grounded in the Fresenius Code of Conduct.
➔ Read more on our Compliance page.

Sustainability Insights
Explore our stories to learn what sustainability looks like in our daily operations:
Our commitment to a healthy planet
As a healthcare company, we operate in a special field of tension. On the one hand, we want to do our best to reduce potentially adverse environmental impacts to a minimum. On the other hand, we must never lose sight of the strict requirements that are placed on patients’ safety and hygiene. Our aim is to promote human health while further reducing our ecological footprint.
People need a healthy home – today and tomorrow. By reducing the environmental impact of our activities, we want to play our part in mitigating climate change and conserving natural resources.

The Planet Dimension of our Sustainability Framework encompasses the following focus topics:
We have a clear goal: to decarbonize our operations and our value chain.
Fresenius manufactures medical products and operates healthcare facilities, which inevitably results in energy consumption and associated greenhouse gas emissions. In production, for example, the machines and containers have to be sterilized, and in our hospitals a wide variety of technical systems are in constant operation. This presents us with special challenges: On the one hand, we want to reduce our energy demand as far as possible and on the other hand, we must always guarantee the safety of patients in our facilities and ensure a stable supply of energy in our production.
We want to live up to our responsibilities and help achieve the goal of the Paris Climate Agreement: Our climate targets aim to limit global warming to 1.5 °C. All our climate protection activities contribute to our long-term objective of attaining net zero by 2050: This requires a company to reduce its avoidable greenhouse gas emissions, while unavoidable emissions must be offset by removing an equivalent amount from the atmosphere and storing it for the long term.
Within our own operations, we are committed to reducing our Scope 1 and Scope 2 emissions by 50% by 2030.
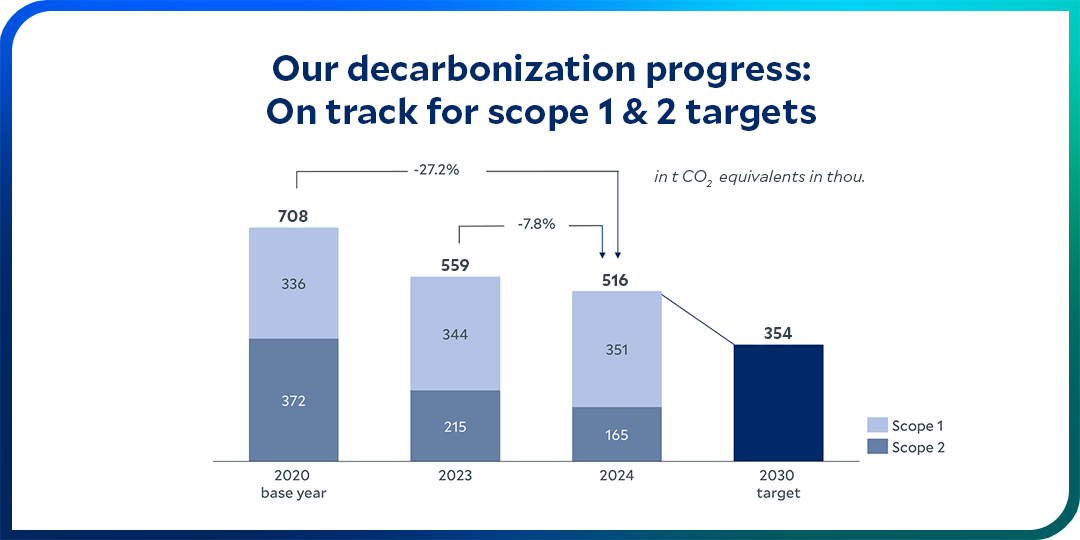
The decarbonization of our value chain is a decisive factor on our long-term path to net zero. Scope 3 emissions include greenhouse gases that are released indirectly in our upstream and downstream value chains – such as from purchased raw materials or at the end of the life of sold products.
Climate change is also a health issue. Rising temperatures, more frequent heatwaves and changing weather patterns directly affect people's health, particularly vulnerable groups such as the elderly, chronically ill, and children. Heat stress can lead to circulatory problems, dehydration and an increased risk of death. At the same time, climate change contributes to the spread of infectious diseases, for example by introducing new vectors such as mosquitoes or contaminating water sources. As a healthcare company, we therefore consider it our responsibility to not only react to the effects of climate change, but also actively contribute to mitigating it. After all, climate protection is health protection.

We place particular emphasis on preserving water quality. In water-stressed areas, we are especially committed to reducing our water withdrawal.

Water is one of our most valuable resources. As a healthcare company, we are dependent on water: We need drinking water of the highest quality to ensure safe patient care. It is crucial for hygiene and well-being. We also use water in the production of our pharmaceutical products – as process water as well as product component. The quality requirements for this water are even higher than those for drinking water.
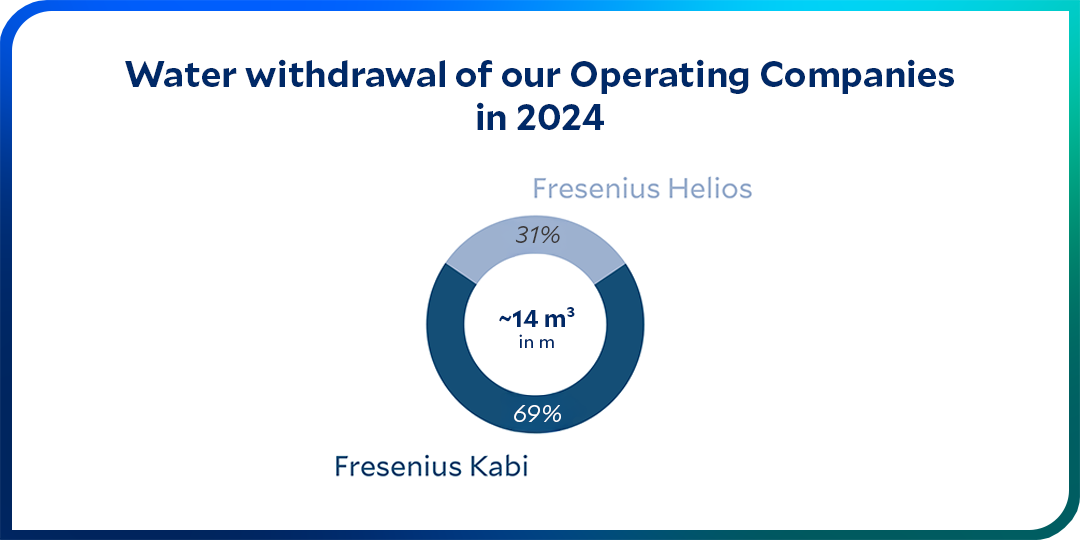
It is our ambition to avoid or minimize any negative impact on the environment that may arise from our direct business operations or from downstream activities. This also includes avoiding unnecessarily polluting the sources from which we obtain water or into which we discharge our wastewater.
At Fresenius, we focus on reducing our waste and embedding circular principles into product design and packaging solutions.
We are committed to conserving natural resources wherever possible. In doing so, we always have to strike a balance between resource efficiency and hygiene requirements. Disposable items are often used in clinics for hygienic reasons. Our options for saving resources are limited here. There are also strict regulations for pharmaceutical packaging. Our most important levers for conserving resources are therefore developing durable and resource-saving products, reusing resources wherever possible, and disposing of waste safely and systematically.


Sustainability Insights
Explore our stories to learn what sustainability looks like in our daily operations:
Excellent care – Human to Human
Our pledge, Committed to Life, underlines our mission: To save lives and improve patients’ health and quality of life. Our employees put this promise into practice every day – whether in direct contact with patients or behind the scenes in administration and production.
People are at the heart of our business activities. We accompany patients through various stages of their lives – sometimes even on a lifelong basis. In doing so, we treat them with respect, as equals, and with a deep understanding of their needs.
We want to promote access to high-quality healthcare and medicine. To this end, we focus on innovative and digital treatment options that enable us to reach even more people.
With the commitment they demonstrate day and night, our employees are a key factor in our success. We want to offer them the best possible working environment, where they can develop and reach their full potential.
Our Outreach
We treated
~ 26 m
patients in our hospitals in 2024.
We served
450 m
patients with our healthcare products in 2024.

The Human Dimension of our Sustainability Framework encompasses these focus topics:
Patient centricity at Fresenius means prioritizing multifaceted patient needs and experiences by providing holistic care through market-leading medical products and services – in inpatient and outpatient settings.
Every year, millions of people entrust us with their most precious asset: their health. With our expertise, we accompany our patients through health and illness: from human to human.
We set out to make our treatments more successful by providing high-quality care. This can help to shorten our patients’ hospital stays, enhance their quality of life – and improve patient satisfaction.
➔ Read more in our Sustainability Statement.
Digitalization plays a crucial role in shaping personalized and efficient processes in our hospitals as well as in the use of our products. We continuously digitize existing processes and introduce new digital workflows. Further, we leverage the capabilities of Artificial Intelligence to enhance the patient experience and expand treatment options, while ensuring its responsible use in accordance with ethical standards, data privacy regulations, and transparency principles.
➔ Read more in our Sustainability Statement.
We strive to provide affordable, high-quality products and services that are accessible and enable significant savings to healthcare systems.
Our range of products and services includes the services of a broad network of clinics as well as high-quality pharmaceuticals and medical devices. We also use digitalization opportunities and develop new forms of therapy. In this way, we aim to reach as many people as possible with our healthcare services and products.
We are dedicated to advancing global health by prioritizing patient well-being and empowering employees. Our growing portfolio includes pharmaceuticals – generics and biosimilars – that offer cost-effective alternatives to originator drugs, and our MedTech and Nutrition products that establish a solid foundation for patient care and recovery.
Ensuring that our products are available is vital to patient care. We continuously invest in our global manufacturing network to maintain a reliable and resilient supply of life-saving therapies. By strengthening our supply infrastructure, we help to prevent disruptions and enhance healthcare delivery worldwide.
➔ Read more in our Sustainability Statement.
Strengthening our purpose-driven performance culture to attract, develop, and retain a motivated workforce is a key priority – because our employees are the foundation of delivering excellent medical care.
Committed to Life – our nearly 180,000 employees put this promise into practice every day: whether in direct contact with patients, relatives, and business partners or behind the scenes in administration and production.
As an employer, it is our responsibility to provide good and safe working conditions for our employees. We seek direct interaction with our employees – because their wide variety of experiences and views help us to constantly improve. Providing our employees with the best possible support in the various phases of their careers while also promoting their commitment and development is very important to us.

Our Team
179884
people were employed by the Fresenius Group in 2024.
41.1
– was the average age of our employees in 2024.

Sustainability Insights
Explore our stories to learn what sustainability looks like in our daily operations: