June 27, 2018
Zurich, Switzerland
Credit Suisse – European Medtech & Healthcare Services Day
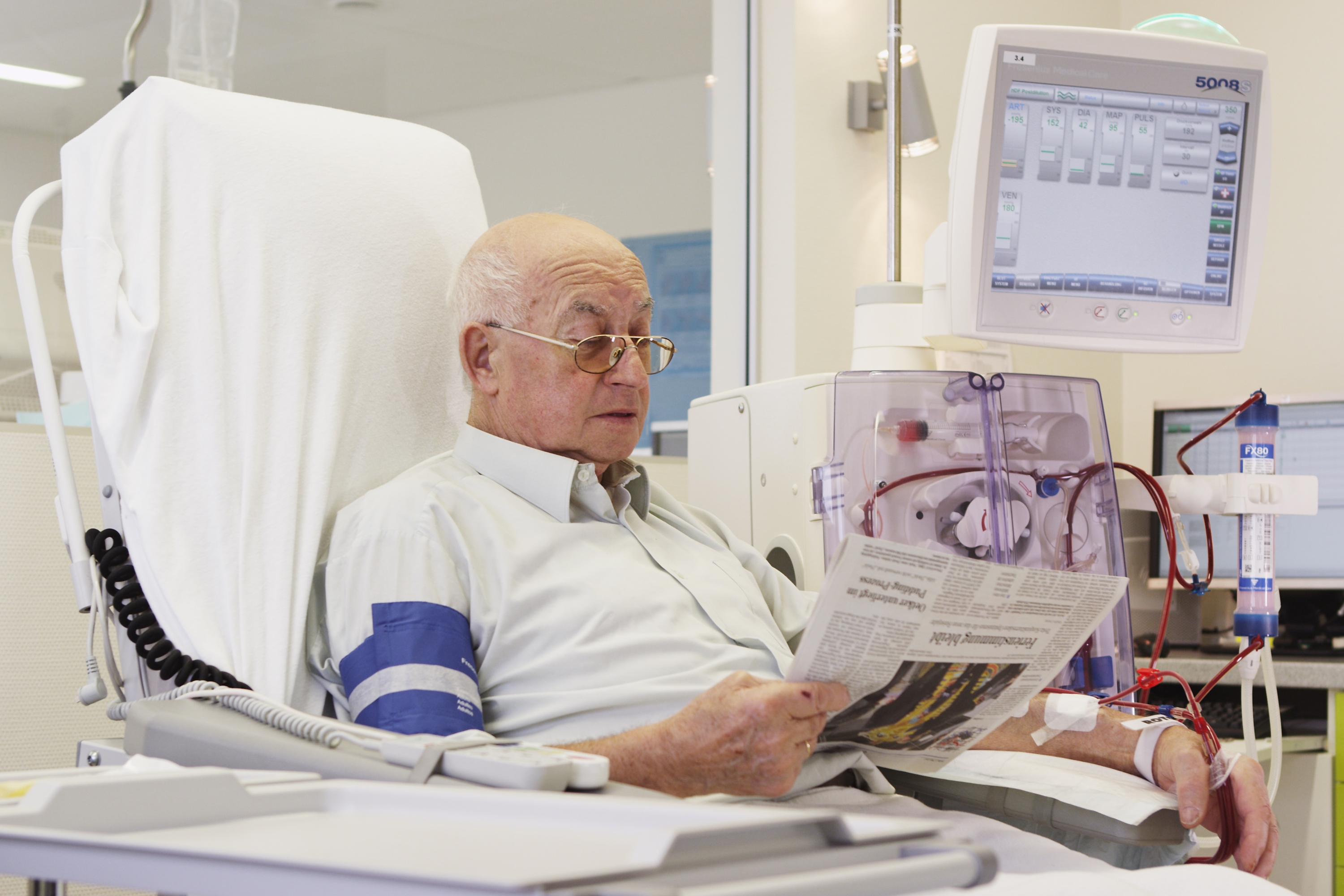
Fresenius Medical Care, the world’s largest provider of dialysis products and services, and Humacyte, Inc., a medical research, discovery and development company, today announced a strategic, global partnership and a $150M USD equity investment. This agreement has the potential to make Humacyte’s investigational human acellular vessel, HUMACYL®, available to more patients worldwide following approval of the product. HUMACYL is currently being investigated for vascular access for hemodialysis and may prove more effective than current synthetic grafts and fistula. Under the terms of the agreement, Fresenius Medical Care will obtain the exclusive global rights to commercialize HUMACYL.
Fresenius Medical Care will be responsible for the marketing, sales and distribution of HUMACYL following approval by the relevant health authorities. In addition, Fresenius Medical Care will make a $150M USD equity investment in Humacyte to gain a 19% fully diluted ownership stake in the company. With the investment, Fresenius Medical Care will have the opportunity to bring transformative clinical innovation in the form of Humacyte’s bioengineered human acellular vessels to the worldwide end stage renal disease (ESRD) patient population following product approval. The transaction is subject to customary closing conditions, including expiration or termination of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and is expected to close in July 2018.
“By partnering with Humacyte, Fresenius Medical Care has an opportunity to offer a dialysis vascular access option with the potential for significant clinical efficacy and safety improvements, including the potential to minimize catheter contact time to the benefit of our patients,” said Franklin Maddux, MD, Chief Medical Officer for Fresenius Medical Care North America. “Our exclusive rights to distribute this innovative technology to dialysis patients worldwide may have significant benefits not only to patients, but health systems as well. With the potential for fewer anticipated complications and interventions compared to synthetic grafts, we may see increased safety for patients and reduced medical and economic burdens to the healthcare system.”
The current vascular access modalities necessary to deliver dialysis treatment include fistulas, grafts and central venous catheters. All three options have limitations. Half of fistulas fail and do not mature in patients, delaying vascular access for dialysis treatment. In the meantime, many patients need a central venous catheter, which significantly increases the risk of infection. Humacyte has developed a novel human tissue-based investigational product, HUMACYL, for patients with ESRD requiring hemodialysis. Compared to an arteriovenous fistula, HUMACYL can be available for use in hemodialysis within weeks and may have an overall higher rate of maturation. It also may offer a more durable, biologic alternative to synthetic grafts.
“This is a transformational milestone for Humacyte, giving us the world’s strongest partner to help bring our product to more patients globally,” said Carrie Cox, CEO and Chairman of Humacyte, Inc. “Our partnership will allow Humacyte to focus on advancing the potential for HUMACYL as a substantial breakthrough in the science of regenerative medicine, and to continue our development of an exciting future pipeline.”
Humacyte’s bioengineered blood vessel is currently in Phase III pivotal trials in the U.S. and Europe, and the company plans to seek regulatory approval in both regions upon completion of the trials.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, risks and uncertainties in research and development and the regulatory approval process; failure to satisfy the conditions to the consummation of the transaction, including the receipt of regulatory approval; regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Without prejudice to its obligations under capital market laws, neither Fresenius Medical Care AG & Co. KGaA nor Humacyte, Inc. undertakes any responsibility to update the forward-looking statements in this release.
June 12, 2018
Rancho Palos Verdes, USA
Goldman Sachs 39th Annual Global Healthcare Conference
June 12 – 13, 2018
More than a year after closing its acquisition of the Spanish hospital operator Quirónsalud, Fresenius Helios foresees strong prospects for further international growth. At a Capital Markets Day in Berlin today, Fresenius provided investors and analysts with an overview of the progress made in the cooperation between Helios Germany and Quirónsalud, which is opening up new growth opportunities in many areas.
Fresenius Helios confirmed the medium-term target for synergies of €50 million annually. Cooperating in laboratory services and joint purchasing has already achieved cost savings in the current business year that should increase to €30 million per year in the medium term. Annual sales synergies of about €20 million are expected from knowledge transfers in medicine, new models for patient care, and digitalization, among other measures.
Quirónsalud has already begun to implement the system developed by Helios Germany for measuring and assessing data on medical quality. To increase transparency and spur individual hospitals to compete on quality, Helios Germany publishes treatment data on the most important and common medical indications for each hospital in direct comparison with national averages compiled by Germany’s Federal Statistical Office. Quirónsalud has already adapted most of these quality indicators. It has also launched peer reviews, collegial exchanges in which the responsible physicians from the individual hospitals advise and consult with each other on questions concerning treatment quality. These exchanges have already led to significant quality improvements at Helios Germany.
The knowledge transfer also extends to digitalization: Quirónsalud’s advanced know-how in patient-oriented uses includes the development of apps, while Helios Germany is particularly strong in IT processes.
Stephan Sturm, CEO of Fresenius, said: “Helios Germany and Quirónsalud are bundling their respective strengths across national borders, exchanging experience and knowledge. This benefits our patients, in Spain as well as in Germany. And it is creating, step by step, the economic prerequisites for the further internationalization of our hospital business. The driving force behind our business success is, and will remain, our clear focus on the well-being of patients. So wherever and whenever we can do more for our patients through closer cooperation between our business segments, we will seize that opportunity.”
Fresenius Helios will also profit from combining the experience gained from the very different health care systems of Spain and Germany. The Spanish system, for its part, allows a great deal of flexibility in care provision models. In Madrid, for example, Quirónsalud has been given responsibility for providing health care to publicly funded patients in designated parts of the city in return for a set reimbursement rate. For patients, however, treatment quality remains the decisive criterion, and assigned patients are still free to choose another hospital. If they do, the cost must be assumed by the hospital that was originally assigned to their care. This increased competition stimulates continuous improvement in areas that are of crucial importance to patients – such as medical quality, service and shorter waiting times. All of these are core competencies of Quirónsalud.
Another area where Fresenius Helios can now draw on its experience from two different health care systems is in classifications of inpatient and outpatient care. The two are strictly separated in Germany, but less so in Spain: Many treatments and after-care procedures that Germans undergo as inpatients are provided to their Spanish counterparts on an outpatient basis – often resulting in significantly shorter hospital stays.
Dr. Francesco de Meo, who is responsible for Fresenius Helios on the Fresenius Management Board, said: “Helios Germany and Quirónsalud are leaders in their home markets, each of which has different reimbursement and care models and health insurance systems. In response, each company has developed its own strengths, which excellently complement each other and can be used to their mutual advantage. We expect this will contribute to higher medical quality and more efficiency, and bring us closer to patients. Thus, together we are building a common base of knowledge and experience that will help us to enter new markets.”
Cooperation with Fresenius Vamed is also being intensified – for example, in procurement, where Fresenius Helios and Fresenius Vamed are now jointly purchasing certain products. In addition, Fresenius Vamed has started providing Quirónsalud hospitals in Spain with technical services and medical technology, as it was already doing for Helios Germany. In Germany, meanwhile, Fresenius Helios and Fresenius Vamed will combine their know-how in hospital construction in order to bundle their competencies in construction and project management.
Earlier this week, the two companies agreed that Fresenius Helios’ inpatient rehabilitation business in Germany will be transferred to Fresenius Vamed on July 1, 2018. This will put Fresenius Helios on a stronger growth footing, with an even clearer focus on the acute-care hospital business and its further internationalization.
Webcast of the event:
Fresenius Helios Capital Markets Day will be available as a webcast on the Internet under: www.fresenius.com/investors-new-developments
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
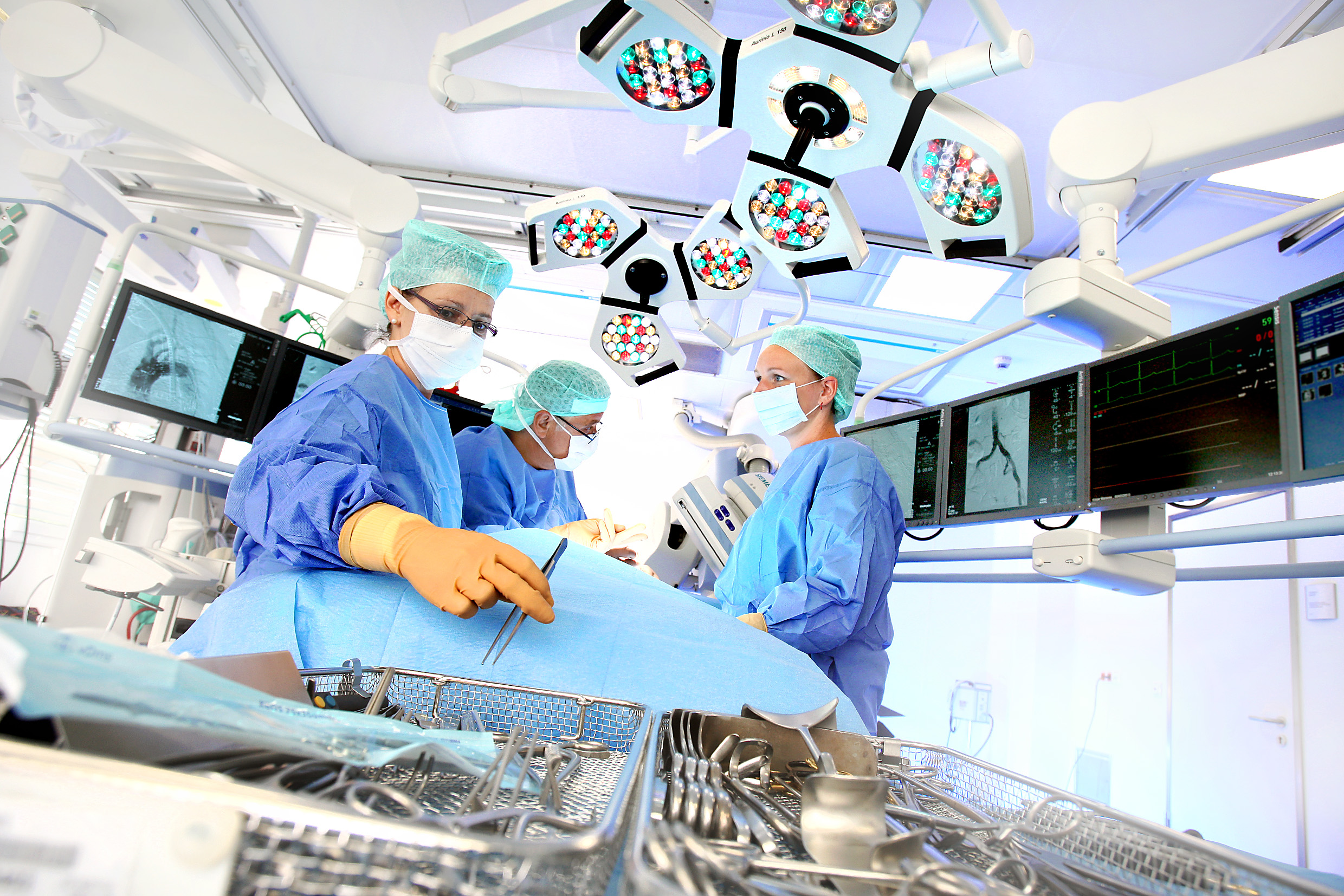
More than a year after closing its acquisition of the Spanish hospital operator Quirónsalud, Fresenius Helios foresees strong prospects for further international growth. At a Capital Markets Day in Berlin today, Fresenius provided investors and analysts with an overview of the progress made in the cooperation between Helios Germany and Quirónsalud, which is opening up new growth opportunities in many areas.
Fresenius Helios confirmed the medium-term target for synergies of €50 million annually. Cooperating in laboratory services and joint purchasing has already achieved cost savings in the current business year that should increase to €30 million per year in the medium term. Annual sales synergies of about €20 million are expected from knowledge transfers in medicine, new models for patient care, and digitalization, among other measures.
Quirónsalud has already begun to implement the system developed by Helios Germany for measuring and assessing data on medical quality. To increase transparency and spur individual hospitals to compete on quality, Helios Germany publishes treatment data on the most important and common medical indications for each hospital in direct comparison with national averages compiled by Germany’s Federal Statistical Office. Quirónsalud has already adapted most of these quality indicators. It has also launched peer reviews, collegial exchanges in which the responsible physicians from the individual hospitals advise and consult with each other on questions concerning treatment quality. These exchanges have already led to significant quality improvements at Helios Germany.
The knowledge transfer also extends to digitalization: Quirónsalud’s advanced know-how in patient-oriented uses includes the development of apps, while Helios Germany is particularly strong in IT processes.
Stephan Sturm, CEO of Fresenius, said: “Helios Germany and Quirónsalud are bundling their respective strengths across national borders, exchanging experience and knowledge. This benefits our patients, in Spain as well as in Germany. And it is creating, step by step, the economic prerequisites for the further internationalization of our hospital business. The driving force behind our business success is, and will remain, our clear focus on the well-being of patients. So wherever and whenever we can do more for our patients through closer cooperation between our business segments, we will seize that opportunity.”
Fresenius Helios will also profit from combining the experience gained from the very different health care systems of Spain and Germany. The Spanish system, for its part, allows a great deal of flexibility in care provision models. In Madrid, for example, Quirónsalud has been given responsibility for providing health care to publicly funded patients in designated parts of the city in return for a set reimbursement rate. For patients, however, treatment quality remains the decisive criterion, and assigned patients are still free to choose another hospital. If they do, the cost must be assumed by the hospital that was originally assigned to their care. This increased competition stimulates continuous improvement in areas that are of crucial importance to patients – such as medical quality, service and shorter waiting times. All of these are core competencies of Quirónsalud.
Another area where Fresenius Helios can now draw on its experience from two different health care systems is in classifications of inpatient and outpatient care. The two are strictly separated in Germany, but less so in Spain: Many treatments and after-care procedures that Germans undergo as inpatients are provided to their Spanish counterparts on an outpatient basis – often resulting in significantly shorter hospital stays.
Dr. Francesco de Meo, who is responsible for Fresenius Helios on the Fresenius Management Board, said: “Helios Germany and Quirónsalud are leaders in their home markets, each of which has different reimbursement and care models and health insurance systems. In response, each company has developed its own strengths, which excellently complement each other and can be used to their mutual advantage. We expect this will contribute to higher medical quality and more efficiency, and bring us closer to patients. Thus, together we are building a common base of knowledge and experience that will help us to enter new markets.”
Cooperation with Fresenius Vamed is also being intensified – for example, in procurement, where Fresenius Helios and Fresenius Vamed are now jointly purchasing certain products. In addition, Fresenius Vamed has started providing Quirónsalud hospitals in Spain with technical services and medical technology, as it was already doing for Helios Germany. In Germany, meanwhile, Fresenius Helios and Fresenius Vamed will combine their know-how in hospital construction in order to bundle their competencies in construction and project management.
Earlier this week, the two companies agreed that Fresenius Helios’ inpatient rehabilitation business in Germany will be transferred to Fresenius Vamed on July 1, 2018. This will put Fresenius Helios on a stronger growth footing, with an even clearer focus on the acute-care hospital business and its further internationalization.
Webcast of the event:
Fresenius Helios Capital Markets Day will be available as a webcast on the Internet under: www.fresenius.com/investors-new-developments
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius is reorganizing the Group’s inpatient rehabilitation business, to create the conditions for the continued growth of Fresenius Helios and Fresenius Vamed. On July 1, 2018, 38 health care facilities and 13 service companies in Germany specializing in inpatient post-acute and nursing care, which are now operated by Fresenius Helios, will be transferred to Fresenius Vamed. This will strengthen Fresenius Vamed’s position as a leading provider of post-acute care in Europe. Fresenius Helios, meanwhile, will focus even more strongly on the acute care hospital business and its continued internationalization.
The transaction has a total volume of €485 million, including assumed net debt of €15 million. It will be financed Group-internally. This year, the inpatient post-acute care business that is being transferred is expected to generate about €460 million in sales and an EBIT of around €37 million.
As part of the transfer, Fresenius Vamed is taking on all of the approximately 7,700 employees of the post-acute care business.
With regard to the respective core competencies of Fresenius Vamed and Fresenius Helios, the two Fresenius business segments will further intensify their cooperation, which has already proven successful in Germany and Spain.
Stephan Sturm, CEO of Fresenius, said: “For Fresenius, post-acute care is and will remain an important component of the treatment we provide to our patients. And now by bundling our great expertise in this area within Fresenius Vamed, we are building a platform for further international growth. We are also putting Fresenius Helios’ on a stronger growth footing, with an even clearer focus on acute care. In addition, we are facilitating even more intensive cooperation between these two business segments, for the benefit of our patients.”
Fresenius Vamed is already a leading post-acute care provider in Austria, Switzerland and the Czech Republic, and entered the British market last year. After the transaction, Fresenius Vamed will have a total of 63 inpatient health care facilities in five European countries.
Even after the transfer of its inpatient post-acute care business to Fresenius Vamed, Fresenius Helios will remain Europe’s largest private hospital operator, with 137 hospitals and about 100,000 employees in Germany and Spain. The strategic focus of Fresenius Helios will remain its acute care hospitals, as well as the outpatient acute care - including preventative medicine - and outpatient post-acute care.
As a consequence of the transfer, Fresenius is adjusting the 2018 outlook for Fresenius Helios and Fresenius Vamed. Fresenius Helios now expects EBIT growth of 5% to 8% (7% to 10% previously). Its organic sales growth outlook of 3% to 6% remains unchanged. Fresenius Vamed is now expecting EBIT growth of 32% to 37%1 (5% to 10% previously). Fresenius Vamed’s organic sales growth forecast remains unchanged at 5% to 10%.
The transaction will not significantly affect the 2018 business results of the Fresenius Group, which accordingly confirms its guidance2 for the current business year. Group sales are expected to increase by 5% to 8%3 in constant currency. Net income4,5 is expected to grow by 6% to 9% in constant currency. Excluding expenditures for the further development of the biosimilars business, net income4,6 is expected to grow by ~10% to 13% in constant currency.
1 Expected EBIT of the inpatient post-acute care business in H2/2018: ~€20 million 2 Excluding effects of the Akorn, NxStage and Sound Physicians transactions 3 2017 base adjusted for IFRS 15 adoption (deduction of €486 million at Fresenius Medical Care) 4 Net income attributable to shareholders of Fresenius SE & Co. KGaA 5 2017 base: €1,816 million; 2018 before special items (i.e., transaction-related effects); including expenditures for further development of biosimilars business (€43 million after tax in FY/17 and ~€120 million after tax in FY/18) 6 2017 base: €1,859 million; 2018 before special items (i.e., transaction-related effects)
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.

Fresenius is reorganizing the Group’s inpatient rehabilitation business, to create the conditions for the continued growth of Fresenius Helios and Fresenius Vamed. On July 1, 2018, 38 health care facilities and 13 service companies in Germany specializing in inpatient post-acute and nursing care, which are now operated by Fresenius Helios, will be transferred to Fresenius Vamed. This will strengthen Fresenius Vamed’s position as a leading provider of post-acute care in Europe. Fresenius Helios, meanwhile, will focus even more strongly on the acute care hospital business and its continued internationalization.
The transaction has a total volume of €485 million, including assumed net debt of €15 million. It will be financed Group-internally. This year, the inpatient post-acute care business that is being transferred is expected to generate about €460 million in sales and an EBIT of around €37 million.
As part of the transfer, Fresenius Vamed is taking on all of the approximately 7,700 employees of the post-acute care business.
With regard to the respective core competencies of Fresenius Vamed and Fresenius Helios, the two Fresenius business segments will further intensify their cooperation, which has already proven successful in Germany and Spain.
Stephan Sturm, CEO of Fresenius, said: “For Fresenius, post-acute care is and will remain an important component of the treatment we provide to our patients. And now by bundling our great expertise in this area within Fresenius Vamed, we are building a platform for further international growth. We are also putting Fresenius Helios’ on a stronger growth footing, with an even clearer focus on acute care. In addition, we are facilitating even more intensive cooperation between these two business segments, for the benefit of our patients.”
Fresenius Vamed is already a leading post-acute care provider in Austria, Switzerland and the Czech Republic, and entered the British market last year. After the transaction, Fresenius Vamed will have a total of 63 inpatient health care facilities in five European countries.
Even after the transfer of its inpatient post-acute care business to Fresenius Vamed, Fresenius Helios will remain Europe’s largest private hospital operator, with 137 hospitals and about 100,000 employees in Germany and Spain. The strategic focus of Fresenius Helios will remain its acute care hospitals, as well as the outpatient acute care - including preventative medicine - and outpatient post-acute care.
As a consequence of the transfer, Fresenius is adjusting the 2018 outlook for Fresenius Helios and Fresenius Vamed. Fresenius Helios now expects EBIT growth of 5% to 8% (7% to 10% previously). Its organic sales growth outlook of 3% to 6% remains unchanged. Fresenius Vamed is now expecting EBIT growth of 32% to 37%1 (5% to 10% previously). Fresenius Vamed’s organic sales growth forecast remains unchanged at 5% to 10%.
The transaction will not significantly affect the 2018 business results of the Fresenius Group, which accordingly confirms its guidance2 for the current business year. Group sales are expected to increase by 5% to 8%3 in constant currency. Net income4,5 is expected to grow by 6% to 9% in constant currency. Excluding expenditures for the further development of the biosimilars business, net income4,6 is expected to grow by ~10% to 13% in constant currency.
1 Expected EBIT of the inpatient post-acute care business in H2/2018: ~€20 million
2 Excluding effects of the Akorn, NxStage and Sound Physicians transactions
3 2017 base adjusted for IFRS 15 adoption (deduction of €486 million at Fresenius Medical Care)
4 Net income attributable to shareholders of Fresenius SE & Co. KGaA
5 2017 base: €1,816 million; 2018 before special items (i.e., transaction-related effects); including expenditures for further development of biosimilars business (€43 million after tax in FY/17 and ~€120 million after tax in FY/18)
6 2017 base: €1,859 million; 2018 before special items (i.e., transaction-related effects)
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.

