- Results unchanged compared with preliminary figures published on July 27, 2022
- Business development impacted by unprecedented U.S. labor market situation and worsening macroeconomic environment driving cost inflation and supply chain disruptions
- Meaningful decline in COVID-19-related excess mortality
- Solid support by positive exchange rates
- FME25: transformation to new operating model and savings generation on track
Decline in COVID-19-related excess mortality
In the second quarter of 2022, COVID-19-related excess mortality among Fresenius Medical Care’s patients declined and amounted to approximately 300 (Q3 2021: ~2,900; Q4 2021: ~2,000; Q1 2022: ~2,4003). Thus, excess mortality accumulated to approximately 7,600 patients over the past twelve months and to approximately 23,000 since the start of the pandemic.
While excess mortality sequentially declined in line with the Company’s projections, infection rates remained on a high level resulting in a continued need and costs for isolation clinics and shifts as well as personal protective equipment.
The overall estimated adverse effect of accumulated excess mortality on organic growth in the Health Care Services business amounted to around 260 basis points in the second quarter.
1 Special items include costs related to the FME25 program, the impact of the war in Ukraine, the impact of hyperinflation in Turkiye, the remeasurement effect on the fair value of the investment in Humacyte, Inc. (Humacyte investment remeasurement) and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance. These items are excluded to ensure comparability of the figures presented with the Company’s financial targets which have been defined excluding special items.
2 Attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
3 Historical excess mortality updated for late entries
Increased headwinds from labor and inflation
The unprecedented U.S. labor market challenges materially worsened in the second quarter. For Fresenius Medical Care, this resulted in meaningfully higher than assumed wage inflation, surcharges, retention payments and additional costs for contract labor to contain the increasing staff shortages. Despite these additional investments in labor, including application of monies received from the U.S. government's Provider Relief Fund, staff shortages and turnover rates have continued to increase. The Company’s growth in the second quarter was affected by the number of clinics with constrained ability to accept new patients for treatment.
The already existing challenging macroeconomic environment has further significantly deteriorated in the second quarter as well, driving accelerated non-wage cost inflation. This has been exacerbated by the ongoing war in Ukraine and its global economic impact and results in higher logistics costs, raw material and energy prices as well as further supply chain disruptions.
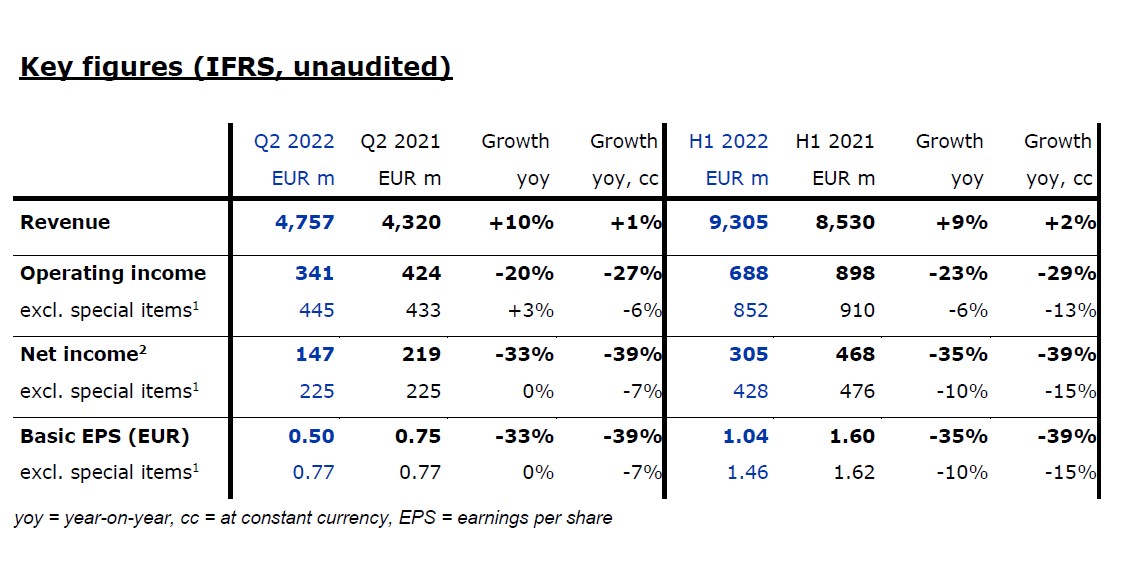
Revenue increased by 10% to EUR 4,757 million (+1% at constant currency, +0% organic) in the second quarter.
Health Care Services revenue grew by 11% to EUR 3,782 million (+1% at constant currency, +0% organic). Growth at constant currency was mainly driven by contributions from acquisitions.
Health Care Products revenue increased by 6% to EUR 975 million (+1% at constant currency, +1% organic). Constant currency growth was mainly driven by higher sales of in-center disposables, partially offset by lower sales of acute cardiopulmonary products.
In the first half, revenue grew by 9% to EUR 9,305 million (+2% at constant currency, +1% organic). Health Care Services revenue increased by 10% to EUR 7,389 million (+2% at constant currency, +1% organic); Health Care Products revenue grew by 6% to EUR 1,916 million (+2% at constant currency, +2% organic).
Operating income decreased by 20% to EUR 341 million (-27% at constant currency) in the second quarter, resulting in a margin of 7.2% (Q2 2021: 9.8%). Operating income excluding special items, i.e. costs incurred for FME25, the impacts related to the war in Ukraine, the impact of hyperinflation in Turkiye, and the remeasurement effect on the fair value of the investment in Humacyte, Inc. (Humacyte investment remeasurement), increased by 3% to EUR 445 million (-6% at constant currency), resulting in a margin of 9.4% (Q2 2021: 10.0%). At constant currency, the decline was mainly due to higher labor costs as well as inflationary and supply chain cost increases. This was partially offset by Provider Relief Funding received from the U.S. government to compensate for certain COVID-19-related costs.
In the first half, operating income declined by 23% to EUR 688 million (-29% at constant currency), resulting in a margin of 7.4% (H1 2021: 10.5%). Excluding special items, operating income decreased by 6% to EUR 852 million (-13% at constant currency), resulting in a margin of 9.2% (H1 2021: 10.7%).
Net income2 decreased by 33% to EUR 147 million (-39% at constant currency). Excluding special items, net income2 was stable and amounted to EUR 225 million (-7% at constant currency). At constant currency, the decline was mainly due to the mentioned negative effects on operating income. Basic earnings per share (EPS) decreased by 33% to EUR 0.50 (-39% at constant currency). Excluding special items, EPS was stable and amounted to EUR 0.77 (-7% at constant currency).
In the first half, net income2 declined by 35% to EUR 305 million (-39% at constant currency). Excluding special items, net income2 decreased by 10% to EUR 428 million
(-15% at constant currency). EPS decreased by 35% to EUR 1.04 (-39% at constant currency). Excluding special items, EPS declined by 10% to EUR 1.46 (-15% at constant currency).
Regional developments
In North America, revenue increased by 12% to EUR 3,294 million (-1% at constant currency, -2% organic) in the second quarter. At constant currency, this was mainly due to a decline in organic growth – which was driven by COVID-19 as well as by declines in co-insurance, increases in patient choice of higher deductibles plans, and lower than expected collections in aged accounts receivable in the Health Care Services business – and due to lower sales of in-center disposables, machines for chronic treatment, renal pharmaceuticals and home hemodialysis products. These effects were only partially offset by contributions from acquisitions. In the first half, revenue grew by 10% to
EUR 6,464 million (+0% at constant currency, -1% organic).
Operating income in North America decreased by 14% to EUR 340 million (-24% at constant currency) in the second quarter, resulting in a margin of 10.3% (Q2 2021: 13.5%). At constant currency, the decline in operating income was mainly due to higher labor costs, the Humacyte investment remeasurement, declines in co-insurance, increases in patient choice of higher deductibles plans, and lower than expected collections in aged accounts receivable, the impact of COVID-19, as well as inflationary and supply chain costs. This was partially offset by provider relief funding received from the U.S. government to compensate for certain COVID-19-related costs. In the first half, operating income declined by 19% to EUR 644 million (-26% at constant currency), resulting in a margin of 10.0% (H1 2021: 13.6%).
Revenue in the EMEA region increased by 5% to EUR 727 million in the second quarter (+7% at constant currency, +6% organic). At constant currency, this was mainly due to organic growth in Health Care Services and Health Care Products, both including the effects of hyperinflation in Turkiye. Growth in Health Care Products was driven by higher sales of in-center disposables, machines for chronic treatment and renal pharmaceuticals, partially offset by lower sales of acute cardiopulmonary products. In the first half, revenue grew by 3% to EUR 1,401 million (+5% at constant currency, +4% organic).
Operating income in EMEA decreased by 19% to EUR 60 million (-18% at constant currency) in the second quarter, resulting in a margin of 8.2% (Q2 2021: 10.6%). At constant currency, the decline in operating income was mainly due to inflationary cost increases, the impact of hyperinflation in Turkiye and costs associated with the FME25 program, partially offset by favorable currency transaction effects. In the first half, operating income declined by 21% to EUR 121 million (-18% at constant currency), resulting in a margin of 8.6% (H1 2021: 11.2%).
In Asia-Pacific, revenue increased by 6% to EUR 516 million (+2% at constant currency, +2% organic) in the second quarter. At constant currency, this was mainly driven by organic growth in the Health Care Services business. In the first half, revenue increased by 7% to EUR 1,023 million (+3% at constant currency, +3% organic).
Operating income decreased by 16% to EUR 71 million (-16% at constant currency) in the second quarter, resulting in a margin of 13.8% (Q2 2021: 17.3%). At constant currency, the decline in operating income was mainly due to the unfavorable impact of growth in lower margin businesses and inflationary cost increases. In the first half, operating income was stable and amounted to EUR 170 million (-1% at constant currency), resulting in a margin of 16.6% (H1 2021: 17.7%).
Latin America revenue increased by 21% to EUR 207 million (+17% at constant currency, +18% organic) in the second quarter, mainly driven by organic growth in the Health Care Services business, as well as higher sales of in-center disposables and machines for chronic treatment. In the first half, revenue grew by 18% to EUR 391 million (+16% at constant currency, +17% organic).
Operating income decreased to EUR -6 million in the second quarter, resulting in a margin of -3.0% (Q2 2021: 1.5%). At constant currency, the decline in operating income was mainly due to inflationary cost increases and unfavorable foreign currency transaction effects, partially offset by lower bad debt expense. In the first half, operating income decreased by 46% to EUR 5 million (-71% at constant currency), resulting in a margin of 1.3% (H1 2021: 2.8%).
Cash flow development
In the second quarter, Fresenius Medical Care generated EUR 751 million of operating cash flow (Q2 2021: EUR 921 million), resulting in a margin of 15.8% (Q2 2021: 21.3%). The decrease was mainly due to an unfavorable development of days sales outstanding as well as a decrease in net income2, partially offset by U.S. government relief funding. In the first half, operating cash flow amounted to EUR 910 million (H1 2021: EUR 1,129 million), resulting in a margin of 9.8% (H1 2021: 13.2%).
Free cash flow4 amounted to EUR 582 million (Q2 2021: EUR 720 million) in the second quarter, resulting in a margin of 12.2% (Q2 2021: 16.7%). In the first half, free cash flow amounted to EUR 581 million (H1 2021: EUR 749 million), resulting in a margin of 6.2% (H1 2021: 8.8%).
4 Net cash provided by / used in operating activities, after capital expenditures, before acquisitions, investments, and dividends
Patients, clinics and employees
As of June 30, 2022, Fresenius Medical Care treated 345,687 patients in 4,163 dialysis clinics worldwide and had 123,153 employees (full-time equivalents) globally, compared to 123,538 employees as of June 30, 2021.
FME25 update
With savings of EUR 26 million in the first half of the year, Fresenius Medical Care is on track to achieve its savings target of EUR 40-70 million in 2022 as part of the FME25 transformation program. Key achievements in the first half of the year include the announcement of the first two leadership levels below the Management Board and the corresponding organizational structure in line with the future operating model. The Company has also made significant progress in the transformation of global G&A functions. In addition to the ongoing and already identified FME25 measures, Fresenius Medical Care is currently in the process of reviewing potential additional initiatives in both designated operating segments (Care Delivery and Care Enablement) as part of the transformation program.
Outlook
As announced on July 27, 2022, Fresenius Medical Care expects revenue to grow at a low single digit percentage rate and net income2 to decline at around a high teens percentage range. Revenue and net income guidance are both on a constant currency basis and before special items5.
These targets are based on the following operating income relevant assumptions:
- Macro-economic inflation and supply chain costs of around EUR 220 million
- COVID-19: impact of accumulated excess mortality of around EUR 100 million
- U.S. labor costs expected to be around EUR 100 million, net of support from U.S. Provider Relief Fund, in excess of the 3% base wage inflation assumption
- U.S. ballot initiative expense of EUR 20 to 30 million
- Business growth of EUR 70 million
- Personal protective equipment cost reduction of around EUR 20 million
- FME25 savings of EUR 40 to 70 million
- Remeasurement effects on the fair value of investments are expected to be volatile but neutral on a full year basis; for guidance relevant comparison, the Humacyte investment remeasurement is treated as special item
- No meaningful further impact from natural gas shortages or suspension of gas supply to affect manufacturing sites
5 These targets are based on the 2021 results excluding the costs related to FME25 of EUR 49 million (for Net Income). They are in constant currency and exclude special items. Special items include further costs related to FME25, the impact of the war in Ukraine, the impact of hyperinflation in Turkiye, the Humacyte investment remeasurement and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance.
Please refer to our statement of earnings included in the attachments as separate PDF files for a complete overview of the results of the second quarter and first half of 2022. Our 6-K disclosure provides more details.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to COVID-19, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Implementation of measures as presented herein may be subject to information and consultation procedures with works councils and other employee representative bodies, as per local laws and practice. Consultation procedures may lead to changes on proposed measures.
- Business development marked by significantly worsening headwinds at Fresenius Medical Care and increased macroeconomic challenges
- Fresenius Medical Care Business development impacted by unprecedented U.S. labor market situation and worsening macroeconomic environment
- Fresenius Kabi with solid organic sales growth despite tough prior-year-quarter
- Fresenius Helios with continued good admissions growth in Germany and Spain
- Fresenius Vamed still impacted by ongoing headwinds; service business supported by increasing elective treatment activity
- Cost and efficiency program evolving according to plan
If no timeframe is specified, information refers to Q2/2022.
1 Before special items, Q1/22 restated following remeasurement Humacyte investment
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 Excluding Ivenix acquisition
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
FY/22 Group guidance
As announced on July 27, 2022, at constant currency, the Company now anticipates Group sales1 to grow in a low-to-mid single-digit percentage range (previously: mid-single digit percentage range) and Group net income2,3 to decline in a low-to-mid single-digit percentage range (previously: increase in a low-single-digit percentage range).
Without the already closed acquisitions of Ivenix and the already completed acquisition of a majority stake in mAbxience as well as any further potential acquisitions, Fresenius expects the net debt/EBITDA4 ratio (December 31, 2021: 3.51x5) to be slightly above the top end of the self-imposed target corridor of 3.0x to 3.5x by the end of 2022.
Assumptions for guidance FY/22
Due to the meaningfully increased uncertainty and volatility related to the war in Ukraine, the ongoing impacts of the COVID-19 pandemic, and a rapidly worsening global macro-economic development, Fresenius now expects significantly more pronounced headwinds in 2022 from supply chain disruptions and cost inflation, including energy prices. Furthermore, Fresenius expects significant negative effects from ongoing labor shortages and associated wage inflation, especially at Fresenius Medical Care in the U.S.
The war in Ukraine is directly and indirectly affecting Fresenius Group operations. The direct adverse effects of the war amounted to €20 million at net income6 level of Fresenius Group in H1/22 and are treated as a special item. Fresenius will continue to closely monitor the potential further consequences of the war, including balance sheet valuations. The guidance does not consider a significant disruption of gas or electricity supplies in Europe.
COVID-19 will continue to impact Fresenius Group operations in 2022. An unlikely but possible significant deterioration of the situation triggering containment measures that could have a significant and direct impact on the health care sector without any appropriate compensation is not reflected in the Group’s FY/22 guidance.
Furthermore, the updated assumptions for Fresenius Medical Care's FY/22 guidance are also fully applicable to Fresenius Group's FY/22 guidance. All of these assumptions are subject to considerable uncertainty. The acquisitions of Ivenix and of the majority stake in mAbxience as well as any further potential acquisitions remain excluded from guidance.
1 FY/21 base: €37,520 million
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 FY/21 base: €1,867 million; before special items; FY/22: before special items
4 At LTM average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures; excluding further potential acquisitions; before special items; including lease liabilities
5 At LTM average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures; before special items; including lease liabilities
6 Net income attributable to shareholders of Fresenius SE & Co. KGaA
For a detailed overview of special items please see the reconciliation tables on pages 20-23.
Cost and efficiency program
The Group’s cost and efficiency program is running according to plan and Fresenius confirms its increased savings targets provided in February 2022 of at least €150 million p.a. after tax and minority interest in 2023. For the years thereafter, a further significant increase in sustainable cost savings is expected.
3% sales increase in constant currency
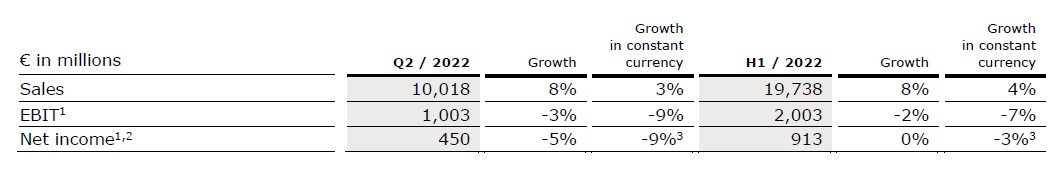
Group sales increased by 8% (3% in constant currency) to €10,018 million (Q2/21: €9,246 million). Organic growth was 2%. Acquisitions/divestitures contributed net 1% to growth. Currency translation increased sales growth by 5%. Excluding estimated COVID-19 effects1, Group sales growth would have been 2% to 3% in constant currency (Q2/21: 6% to 7%).
In H1/22, Group sales increased by 8% (4% in constant currency) to €19,738 million (H1/21: €18,230 million). Organic growth was 3%. Acquisitions/divestitures contributed net 1% to growth. Currency translation increased sales growth by 4%. Excluding estimated COVID-19 effects1, Group sales growth would have been 4% to 5% in constant currency (H1/21: 5% to 6%).
9% net income2,3,4 decline in constant currency
Group EBITDA before special items remained stable (-6% in constant currency) at €1,682 million (Q2/212: €1,674 million). Reported Group EBITDA was €1,528 million (Q2/21: €1,662 million).
In H1/22, Group EBITDA before special items increased by 1% (-4% in constant currency) to €3,344 million (H1/212: €3,305 million). Reported Group EBITDA was €3,123 million (H1/21: €3,290 million).
Group EBIT before special items decreased by 3% (-9% in constant currency) to €1,003 million (Q2/212: €1,033 million). The decrease was mainly driven by worsened labor shortages and related meaningfully increased wage inflation at Fresenius Medical Care in the U.S. as well as elevated material and logistic costs. The EBIT margin before special items was 10.0% (Q2/212: 11.2%). Reported Group EBIT was €845 million (Q2/21: €1,021 million).
In H1/22, Group EBIT before special items decreased by 2% (-7% in constant currency) to €2,003 million (H1/212: €2,042 million). The EBIT margin before special items was 10.1% (H1/212: 11.2%). Reported Group EBIT was €1,747 million (H1/21: €2,027 million).
1 For estimated COVID-19 effects please see table on page 18 of the pdf.
2 Before special items
3 Net income attributable to shareholders of Fresenius SE & Co. KGaA
4 Excluding Ivenix acquisition
Group net interest before special items improved to -€116 million (Q2/21: -€121 million) mainly due to positive one-time effects despite an increased interest rate environment. Reported Group net interest also improved to -€116 million (Q2/21: -€121 million). In H1/22, Group net interest before special items improved to -€235 million (H1/211: -€258 million). Reported Group net interest also improved to -€234 million (H1/21: -€258 million).
Group tax rate before special items was 23.0% (Q2/211: 21.5%) while the reported Group tax rate was 22.6% (Q2/21: 21.3%). In H1/22, Group tax rate before special items was 22.9% (H1/211: 22.1%) while the reported Group tax rate was 23.1% (H1/2021: 22.0%).
Noncontrolling interests before special items were -€233 million (Q2/211: -€241 million) of which 90% were attributable to the noncontrolling interests in Fresenius Medical Care. Reported noncontrolling interests were -€181 million (Q2/21: -€237 million). In H1/22, Noncontrolling interests before special items were -€451 million (H1/211: -€478 million) of which 89% were attributable to the noncontrolling interests in Fresenius Medical Care. Reported noncontrolling interests were -€367 million (H1/21: -€473 million).
Group net income2 before special items decreased by 5% (-9%3 in constant currency) to €450 million (Q2/211: €475 million). The decrease was mainly driven by worsened labor shortages and related meaningfully increased wage inflation at Fresenius Medical Care in the U.S. as well as elevated material and logistic costs. Excluding estimated COVID-19 effects4, Group net income2 before special items was -16% to -12% in constant currency (Q2/21: 10% to 14%). Reported Group net income2 decreased to €383 million (Q2/21: €471 million).
In H1/22, Group net income2 before special items remained stable (-3%3 in constant currency) at €913 million (H1/211: €911 million). Excluding estimated COVID-19 effects4, Group net income2 before special items was -10% to -6% in constant currency (H1/21: 4% to 8%). Reported Group net income2 decreased to €796 million (H1/21: €906 million).
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 Excluding Ivenix acquisition
4 For estimated COVID-19 effects please see table on page 18 of the pdf.
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
Earnings per share1 before special items decreased by 6% (-11% in constant currency) to €0.80 (Q2/212: €0.85). Reported earnings per share1 were €0.68 (Q2/21: €0.84). In H1/22, earnings per share1 before special items remained stable (-4% in constant currency) at €1.63 (H1/212: €1.63). Reported earnings per share1 were €1.42 (H1/21: €1.62).
Continued investment in growth
Spending on property, plant and equipment was €419 million corresponding to 4% of sales (Q2/21: €509 million; 6% of sales). These investments served primarily for the modernization and expansion of dialysis clinics, production facilities as well as hospitals and day clinics. In H1/22, spending on property, plant and equipment was €757 million corresponding to 4% of sales (H1/21: €893 million; 5% of sales).
Total acquisition spending was €291 million (Q2/21: €491 million), mainly for the acquisition of Ivenix by Fresenius Kabi and dialysis clinics at Fresenius Medical Care. In H1/22, total acquisition spending was €453 million (H1/21: €640 million).
Cash flow development
Group operating cash flow decreased to €1,017 million (Q2/21: €1,451 million) with a margin of 10.2% (Q2/21: 15.7%), mainly driven by working capital build-up from higher raw material inventories and receivables, among others, as well as phasing effects. Free cash flow before acquisitions and dividends decreased to €581 million (Q2/21: €952 million). Free cash flow after acquisitions and dividends decreased to -€391 million (Q2/21: -€359 million).
In H1/22, Group operating cash flow decreased to €1,118 million (H1/21: €2,103 million) with a margin of 5.7% (H1/21: 11.5%). Free cash flow before acquisitions and dividends decreased to €326 million (H1/21: €1,193 million). Free cash flow after acquisitions and dividends decreased to -€794 million (H1/21: -€242 million).
1 Net income attributable to shareholders of Fresenius SE & Co. KGaA
2 Before special items
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
Solid balance sheet structure
Group total assets increased by 6% (1% in constant currency) to €76,112 million (Dec. 31, 2021: €71,962 million) given currency translation effects and the expansion of business activities. Current assets increased by 8% (4% in constant currency) to €18,818 million (Dec. 31, 2021: €17,461 million), mainly driven by the increase of trade accounts receivables. Non-current assets increased by 5% (1% in constant currency) to €57,294 million (Dec. 31, 2021: €54,501 million).
Total shareholders’ equity increased by 9% (3% in constant currency) to €32,033 million (Dec. 31, 2021: €29,288 million). The equity ratio was 42.1% (Dec. 31, 2021: 40.7%).
Group debt increased by 4% (2% in constant currency) at €28,368 million (Dec. 31, 2021: € 27,155 million). Group net debt increased by 8% (5% in constant currency) to € 26,239 million (Dec. 31, 2021: € 24,391 million).
As of June 30, 2022, the net debt/EBITDA ratio increased to 3.72x1,2 (Dec. 31, 2021: 3.51x1,2) mainly driven by dividend payments, lower EBITDA contribution as well as acquisition spending. The net debt/EBITDA as of June 30, 2022 excluding the already closed acquisition of Ivenix was 3.681,2.
1 At LTM average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures
2 Before special items
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
Increased number of employees
As of June 30, 2022, the Fresenius Group had 318,647 employees worldwide (December 31, 2021: 316,078).
Business Segments
Fresenius Medical Care (Financial data according to Fresenius Medical Care press release)
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases. As of June 30, 2022, Fresenius Medical Care was treating 345,687 patients in 4,163 dialysis clinics. Along with its core business, the Renal Care Continuum, the company focuses on expanding in complementary areas and in the field of critical care.
- Business development impacted by unprecedented U.S. labor market situation and worsening macroeconomic environment driving cost inflation and supply chain disruptions
- Meaningful decline in COVID-19-related excess mortality
- Solid support by positive exchange rates
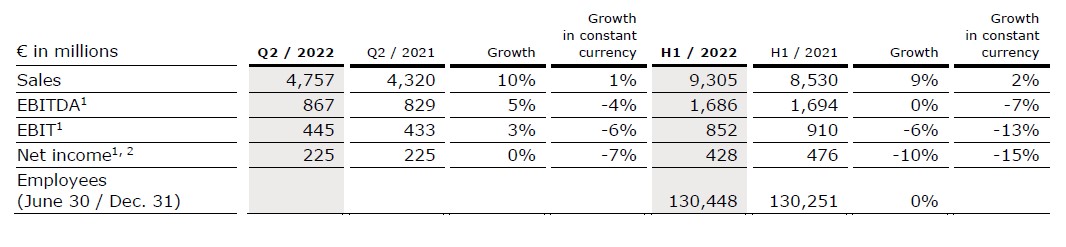
Sales increased by 10% (1% in constant currency) to €4,757 million (Q2/21: €4,320 million). Organic growth was 0%. Currency translation increased sales growth by 9%. In H1/22, sales increased by 9% (2% in constant currency) to €9,305 million (H1/21: €8,530 million). Organic growth was 1%. Currency translation increased sales growth by 7%.
EBIT decreased by 20% (-27% in constant currency) to €341 million (Q2/21: €424 million) resulting in a margin of 7.2% (Q2/21: 9.8%). EBIT before special items, i.e. costs incurred for FME25, the impacts related to the war in Ukraine, the impact of hyperinflation in Turkey and the remeasurement effect on the fair value of the investment in Humacyte, Inc. increased by 3% (-6% in constant currency) to €445 million (Q2/21: €433 million), resulting in a margin1 of 9.4% (Q2/21: 10.0%). At constant currency, the decline was mainly due to higher labor costs as well as inflationary and supply chain cost increases. This was partially offset by Provider Relief Funding received from the U.S. government to compensate for certain COVID-19-related costs.
1 Before special items
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
In H1/22, EBIT decreased by 23% (-29% in constant currency) to €688 million (H1/21: €898 million) resulting in a margin of 7.4% (H1/21: 10.5%). EBIT6 before special items decreased by 6% (-13% in constant currency) to €852 million (H1/21: €910 million), resulting in a margin1 of 9.2% (H1/21: 10.7%).
Net income2 decreased by 33% (-39% in constant currency) to €147 million (Q2/21: €219 million). Net income2 before special items remained stable (-7% in constant currency) at €225 million (Q2/21: €225 million) mainly due to the mentioned negative effects on operating income.
In H1/22, net income2 decreased by 35% (-39% in constant currency) to €305 million (H1/21: €468 million). Net income2 before special items decreased by 10% (-15% in constant currency) to €428 million (H1/21: €476 million).
Operating cash flow was €751 million (Q2/21: €921 million) with a margin of 15.8% (Q2/21: 21.3%). The decrease was mainly due to an unfavorable development of days sales outstanding as well as a decrease in net income2, partially offset by U.S. government relief funding. In H1/22, operating cash flow was €910 million (H1/21: €1,129 million) with a margin of 9.8% (H1/21: 13.2%).
As announced on July 27, 2022, Fresenius Medical Care expects revenue3 to grow at a low single digit percentage rate and net income2,4 to decline at around a high teens percentage range.
Revenue and net income guidance are both on a constant currency basis and before special items5.
Given the uncertain labor situation and macro-economic inflationary environment and the substantially reduced earnings base compared to 2020, Fresenius Medical Care does not expect today to be able to achieve the meaningfully higher compounded annual average increases that would now be needed to accomplish its 2025 targets. Against this background, Fresenius Medical Care has cut its financial targets for FY 2022 and withdrawn its 2025 targets.
For further information, please see Fresenius Medical Care’s press release at www.freseniusmedicalcare.com.
1 Before special items
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
3 FY/21 base: €17,619 million
4 FY/21 base: €1,018 million, before special items; FY/22 before special items
5 These targets are based on the 2021 results excluding the costs related to FME25 of EUR 49 million (for Net Income). They are in constant currency and exclude special items. Special items include further costs related to FME25, the impact of the War in Ukraine, the impact of Hyperinflation in Turkey, the Humacyte investment remeasurement and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance.
For a detailed overview of special items please see the reconciliation tables on pages 20-23.
Fresenius Kabi
Fresenius Kabi offers intravenously administered generic drugs, clinical nutrition and infusion therapies for seriously and chronically ill patients in the hospital and outpatient environments. The company is also a leading supplier of medical devices and transfusion technology products. In the biosimilars business, Fresenius Kabi develops products with a focus on oncology and autoimmune diseases.
- North America with solid organic sales growth despite macroeconomic headwinds
- Asia-Pacific impacted by price pressure from NVBP tenders in China
- Biosimilars business progressing well; completing acquisition of majority stake in mAbxience
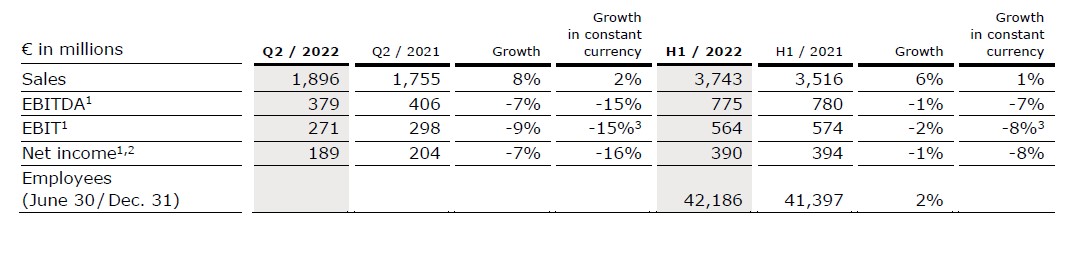
Sales increased by 8% (2% in constant currency) to €1,896 million (Q2/21: €1,755 million). Organic growth was 2%. In H1/22, sales increased by 6% (1% in constant currency) to €3,743 million (H1/21: €3,516 million). Organic growth was 1%. Positive currency translation effects of 6% in Q2/22 and 5% in H1/22 were mainly related to the U.S. dollar and Chinese yuan.
Sales in North America increased by 16% (organic growth: 3%) to €606 million (Q2/21: €522 million). The significant sales growth was mainly driven by positive currency effects while organic growth continued to be impacted a high level of COVID-related absenteeism of production staff, ongoing competitive pressure and supply chain challenges. In H1/22, sales in North America increased by 10% (organic growth: 0%) to €1,185 million (H1/21: €1,080 million).
Sales in Europe increased by 4% (organic growth: 4%) to €658 million (Q2/21: €634 million) driven by a broad-based positive development and biosimilars. In H1/22, sales in Europe increased by 3% (organic growth: 3%) to €1,298 million (H1/21: €1,260 million).
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 Excluding Ivenix acquisition
For a detailed overview of special items please see the reconciliation tables on pages 20-23.
Sales in Asia-Pacific increased by 4% (organic growth: -4%) to €425 million (Q2/21: €409 million). Organic growth was primarily affected by price pressure from the NVBP (National Volume-Based Procurement) tenders in China while Asia-Pacific ex China showed healthy underlying growth. In H1/22, sales in Asia-Pacific increased by 7% (organic growth: -1%) to €858 million (H1/21: €801 million).
Sales in Latin America/Africa increased by 9% (organic growth: 2%) to €207 million (Q2/21: €190 million), over a high prior-year COVID-19-related base. In H1/22, sales in Latin America/Africa increased by 7% (organic growth: 2%) to €402 million (H1/21: €375 million).
Sales in the Biosimilars business was €29 million. In H1/22, sales in the Biosimilars business was €52 million, consistent with Fresenius Kabi’s expectations. The U.S. Food and Drug Administration (FDA) has accepted for review Fresenius Kabi's Biologics License Application (BLA) for MSB11456, a biosimilar candidate of Actemra®4 (tocilizumab). Moreover, Fresenius Kabi closed the majority stake acquisition of mAbxience Holding S.L., a leading international biopharmaceutical company. The transaction was announced in March 2022. The acquisition significantly strengthens Fresenius Kabi’s footprint in the biopharmaceuticals space. The purchase price will be a combination of c. €495 million upfront payment and milestone payments, strictly tied to the achievement of commercial and development targets.
EBIT1 decreased by 9% (-15%2 in constant currency) to €271 million (Q2/21: €298 million) with an EBIT margin1 of 14.3% (Q2/21: 17.0%). Ongoing competitive pressure, staff shortages, supply chain challenges as well as accelerated input cost inflation weighed on the financial performance. In H1/22, EBIT1 decreased by 2% (-8%2 in constant currency) to €564 million (H1/21: €574 million) with an EBIT margin1 of 15.1% (H1/21: 16.3%).
Net income1,3 decreased by 7% (-16% in constant currency) to €189 million (Q2/21: €204 million). In H1/22, net income1,3 decreased by 1% (-8% in constant currency) to €390 million (H1/21: €394 million).
Operating cash flow decreased to €109 million (Q2/21: €197 million) with a margin of 5.7% (Q2/21: 11.2%), mainly driven by a working capital build-up from e.g. higher raw material inventories. In H1/22, operating cash flow decreased to €242 million (H1/21: €475 million) with a margin of 6.5% (H1/21: 13.5%).
1 Before special items
2 Excluding Ivenix acquisition
3 Net income attributable to shareholders of Fresenius SE & Co. KGaA
4 Actemra® is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
For FY/22, Fresenius Kabi confirms its outlook and expects organic sales1 growth in a low-single-digit percentage range. Constant currency EBIT2 is expected to decline in a high-single- to low-double-digit percentage range. Both sales and EBIT outlook include expected COVID-19 effects. The financial effects from the acquisitions of Ivenix and the majority stake in mAbxience remain excluded from guidance.
Save the date: Fresenius will host a virtual Meet the Management event on its business segment Fresenius Kabi on Friday, October 7, 2022 (virtual event).
1 FY/21 base: €7,193 million
2 FY/21 base: €1,153 million, before special items, FY/22 before special items
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
Fresenius Helios
Fresenius Helios is Europe's leading private hospital operator. The company comprises Helios Germany, Helios Spain and Helios Fertility. Helios Germany operates 87 hospitals, ~130 outpatient centers and 6 prevention centers. Helios Spain operates 50 hospitals, 97 outpatient centers and around 300 occupational risk prevention centers. In addition, the company is active in Latin America with 8 hospitals and as a provider of medical diagnostics. Helios Fertility offers a wide spectrum of state-of-the-art services in the field of fertility treatments.
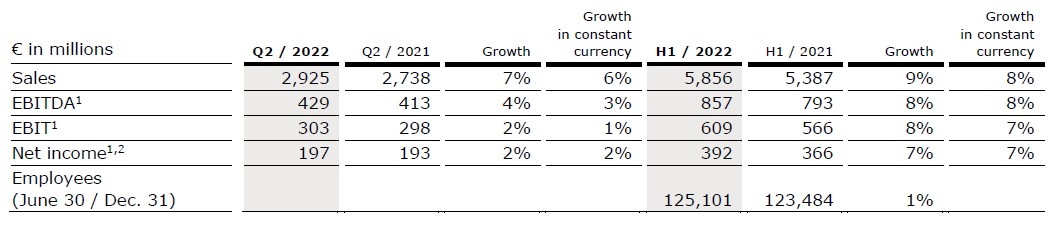
- Fresenius Helios with solid organic growth in Germany and Spain based on increased number of admissions
- Helios Fertility with solid financial performance
Sales increased by 7% (6% in constant currency) to €2,925 million (Q2/21: €2,738 million). Organic growth was 5%. Acquisitions, mainly at Helios Fertility, contributed 1% to sales growth. In H1/22, sales increased by 9% (8% in constant currency) to €5,856 million (H1/21: €5,387 million). Organic growth was 6%. Acquisitions contributed 2% to sales growth.
Sales of Helios Germany increased by 5% (organic growth: 4%) to €1,758 million (Q2/21: €1,675 million), mainly driven by increasing admissions, which are however still below pre-pandemic levels. Acquisitions contributed 1% to sales growth. In H1/22, sales of Helios Germany increased by 6% (organic growth: 5%) to €3,541 million (H1/21: €3,348 million). Acquisitions contributed 1% to sales growth.
Sales of Helios Spain increased by 8% (7% in constant currency) to €1,101 million (Q2/21: €1,020 million). Organic growth of 6% was driven by consistently high activity levels. The hospitals in Latin America also contributed to sales growth. Acquisitions contributed 2% to sales growth. In H1/22, sales of Helios Spain increased by 10% (9% in constant currency) to €2,190 million (H1/21: €1,996 million). Organic growth was 9%.
Sales of the Helios Fertility were €65 million (Q2/21: €42 million). In H1/22, sales of the Helios Fertility were €122 million.
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
EBIT1 increased by 2% (1% in constant currency) to €303 million (Q2/21: €298 million) with an EBIT margin1 of 10.4% (Q2/21: 10.9%). In H1/22, EBIT1 increased by 8% (7% in constant currency) to €609 million (H1/21: €566 million) with an EBIT margin1 of 10.4% (H1/21: 10.5%).
EBIT1 of Helios Germany increased by 1% to €154 million (Q2/21: €152 million) with an EBIT margin1 of 8.8% (Q2/21: 9.1%). COVID-related elevated staff absenteeism weighed on profitability. Inflationary effects had only a small negative impact. In H1/22, EBIT1 of Helios Germany increased by 2% to €308 million (H1/21: €302 million) with an EBIT margin1 of 8.7% (H1/21: 9.0%).
EBIT1 of Helios Spain increased by 1% (0% in constant currency) to €148 million (Q2/21: €147 million) due to an extraordinary high prior-year quarter comp. The Latin American business also showed a good performance. The EBIT margin1 was 13.4% (Q2/21: 14.4%). In H1/22, EBIT1 of Helios Spain increased by 10% (10% in constant currency) to €301 million (H1/21: €273 million). The EBIT margin1 was 13.7% (H1/21: 13.7%).
EBIT1 of Helios Fertility was €7 million with an EBIT margin1 of 10.8% (Q2/21: €5 million). In H1/22, EBIT1 of Helios Fertility was €11 million with an EBIT margin1 of 9.0%.
Net income1,2 increased by 2% (2% in constant currency) to €197 million (Q2/21: €193 million). In H1/22, net income1,2 increased by 7% (7% in constant currency) to €392 million (H1/21: €366 million).
Operating cash flow decreased to €194 million (Q2/21: €223 million) with a margin of
6.6% (Q2/21: 8.1%) following COVID-19-related delays in budget negotiations in Germany. In H1/22, operating cash flow decreased to €58 million (H1/21: €438 million) with a margin of 1.0% (H1/21: 8.1%)
For FY/22, Fresenius Helios confirms its outlook and expects organic sales3 growth in a low- to mid-single-digit percentage range and constant currency EBIT4 growth in a mid-single-digit percentage range. Both sales and EBIT outlook include expected COVID-19 effects.
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 FY/21 base: €10,891 million
4 FY/21 base: €1,127 million, before special items, FY/22 before special items
For a detailed overview of special items please see the reconciliation tables on pages 20-23 of the pdf.
Fresenius Vamed
Fresenius Vamed manages projects and provides services for hospitals and other health care facilities worldwide and is a leading post-acute care provider in Central Europe. The portfolio ranges along the entire value chain: from project development, planning, and turnkey construction, via maintenance and technical management to total operational management.
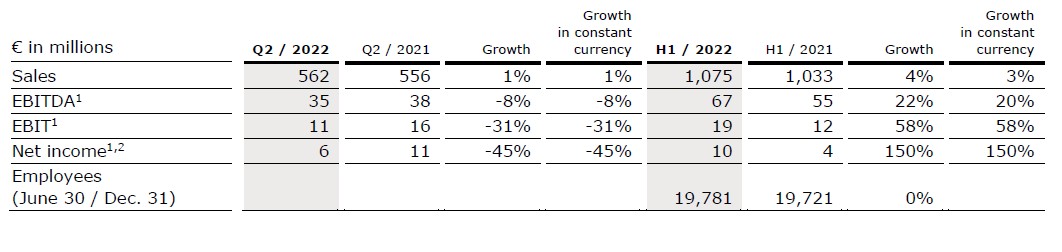
- Project business still marked by the Ukraine war and COVID-19-related headwinds in project execution as well as global supply chain challenges and cost inflation
- Service business supported by increasing elective treatment activity
- Order backlog at all-time high
Sales increased by 1% (1% in constant currency) to €562 million (Q2/21: €556 million). Organic growth was 1%. In H1/22, sales increased by 4% (3% in constant currency) to €1,075 million (H1/21: €1,033 million). Organic growth was 4%.
Sales in the service business increased by 6% (6% in constant currency) to €417 million (Q2/21: €392 million) due to recovering elective treatments. Sales in the project business decreased by 12% (-12% in constant currency) to €145 million (Q2/21: €164 million),
driven by the Ukraine war and COVID-19-related headwinds as well as global supply chain challenges. In H1/22, sales in the service business increased by 9% (8% in constant currency) to €822 million (H1/21: €755 million). Sales in the project business decreased by 9% (-9% in constant currency) to €253 million (H1/21: €278 million).
EBIT1 decreased by 31% to €11 million (Q2/21: €16 million) with an EBIT margin1 of 2.0% (Q2/21: 2.9%) driven by the Ukraine war and COVID-19-related headwinds as well as global supply chain challenges. In H1/22, EBIT1 increased by 58% to €19 million (H1/21: €12 million) with an EBIT margin1 of 1.8% (H1/21: 1.2%).
1 Before special items
2 Net income attributable to shareholders of VAMED AG
For a detailed overview of special items please see the reconciliation tables on pages 20-23.
Net income1,2 decreased by 45% to €6 million (Q2/21: €11 million). In H1/22, Net income1,2 increased to €10 million (H1/21: €4 million).
Order intake was €253 million (Q2/21: €713 million). In H1/22 order intake was €516 million (H1/21: €851 million). As of June 30, 2022, order backlog was at €3,732 million (December 31, 2021: €3,473 million).
Operating cash flow decreased to €7 million (Q2/21: €58 million) with a margin of 1.2% (Q2/21: 10.4%), due to phasing effects and COVID-19-related delays in the project business as well as some working capital build-ups. In H1/22, operating cash flow decreased to -€38 million (H1/21: €14 million) with a margin of -3.5% (H1/21: 1.4%).
For FY/22, Fresenius Vamed confirms its outlook and expects organic sales3 growth in a high-single to low-double-digit percentage range and constant currency EBIT4 to return to absolute pre-COVID-19 levels (FY/19: €134 million). Both sales and EBIT outlook include expected COVID-19 effects.
1 Before special items
2 Net income attributable to shareholders of VAMED AG
3 FY/21 base: €2,297 million
4 FY/21 base: €101 million, before special items; FY/22 before special items
For a detailed overview of special items please see the reconciliation tables on pages 20-23
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Kabi closed the majority stake acquisition of mAbxience Holding S.L. (“mAbxience”), a leading international biopharmaceutical company. The transaction was announced in March 2022.
The acquisition significantly strengthens Fresenius Kabi’s footprint in the biopharmaceuticals space by broadening the product portfolio and expanding its production network with three state-of-the-art facilities for the production of biologic drug substance. This will enable Fresenius Kabi to cover the entire biopharmaceuticals value chain in the future and create flexible, competitive capacities for the production of the expanded biosimilars portfolio. The additional production capacities are expected to generate significant cost synergies with regard to the company's own biosimilars portfolio. Furthermore, the acquisition enables further expansion in the high-growth CDMO (Contract Development and Manufacturing Organization) market for biologics.
The purchase price will be a combination of c. €495 million upfront payment and milestone payments, strictly tied to the achievement of commercial and development targets. The contractual provisions also include a put / call option scheme regarding the sellers’ and future co-owners’ remaining shares in mAbxience (45%).
Fresenius Medical Care’s financial performance in Q2/22 was significantly impacted by worsened labor shortages and related meaningfully increased wage inflation in the U.S. The further deterioration of the macro-economic environment resulted in accelerated non-wage inflation, particularly higher supply chain costs.
Against this backdrop and growing indications for a persistent unfavorable development of these and other factors, Fresenius Medical Care has revised its outlook for FY/22.
All other Fresenius Group segments confirm their respective outlook for FY/22 for both revenue and EBIT.
However, as a consequence of the development at Fresenius Medical Care, and despite all other Fresenius Group segments confirming their respective outlook for both revenue and EBIT, Fresenius now also revises its Group outlook for FY/22. At constant currency, the Company now anticipates Group sales1 to grow in a low-to-mid single-digit percentage range (previously: mid-single digit percentage range) and Group net income2,3 to decline in a low-to-mid single-digit percentage range (previously: increase in a low-single-digit percentage range).
1FY/21 base: €37,520 million
2Net income attributable to shareholders of Fresenius SE & Co. KGaA
3FY/21 base: €1,867 million; before special items; FY/22: before special items
Stephan Sturm, CEO of Fresenius, said: “As a globally active healthcare group, we, too, have inevitably been impacted by – in many cases massive – cost increases, growing problems in the global supply chains, and staff shortages. And unlike companies in other industries, we cannot simply pass on the resulting cost burdens in the short term by raising our prices. To the extent possible and foreseeable, we factored these burdens into the guidance we provided in February and May. In the meantime, though, it has become apparent that patient-facing healthcare services in the United States are affected even more heavily, hence also Fresenius Medical Care. It will take fortitude and energy to overcome this particularly challenging phase, and I am therefore very pleased that Carla Kriwet will assume her new position as CEO of Fresenius Medical Care quite a bit earlier than initially planned. I am confident that, together with her colleagues, she will find the right solutions and lead Fresenius Medical Care into a successful future.”
“Our goal at Fresenius is, and remains, to create more value: for our patients, our employees and our shareholders,” added Sturm. “We are working tirelessly, guided by our clear strategic priorities, to achieve this. And we continue to see good prospects, despite the current burdens and difficulties resulting from global crises. Not least because, from our strong market positions, we moved early to capitalize on the right trends, such as home dialysis. Healthcare is a market of the future that we want to play an important role in shaping, and where we intend to continue our sustained, profitable growth.”
Assumptions for guidance FY/22
Due to the meaningfully increased uncertainty and volatility related to the war in Ukraine, the ongoing impacts of the COVID-19 pandemic, and a rapidly worsening global macro-economic development, Fresenius now expects significantly more pronounced headwinds in 2022 from supply chain disruptions and cost inflation, including energy prices. Furthermore, Fresenius expects significant negative effects from ongoing labor shortages and associated wage inflation, especially at Fresenius Medical Care in the U.S.
The war in Ukraine is directly and indirectly affecting Fresenius Group operations. The direct adverse effects of the war amounted to €20 million at net income1 level of Fresenius Group in H1/22 and are treated as a special item. Fresenius will continue to closely monitor the potential further consequences of the war, including balance sheet valuations. The guidance does not consider a significant disruption of gas or electricity supplies in Europe.
COVID-19 will continue to impact Fresenius Group operations in 2022. An unlikely but possible significant deterioration of the situation triggering containment measures that could have a significant and direct impact on the health care sector without any appropriate compensation is not reflected in the Group’s FY/22 guidance.
Furthermore, the updated assumptions for Fresenius Medical Care's FY/22 guidance are also fully applicable to Fresenius Group's FY/22 guidance.All of these assumptions are subject to considerable uncertainty. The acquisition of Ivenix and the announced acquisition of the majority stake in mAbxience as well as any further potential acquisitions remain excluded from guidance.
Group medium-term targets
As a result of the updated expectations for FY/22, Fresenius now believes its medium-term net income1 target is no longer achievable. Fresenius had expected Group organic net income1 growth to be at the bottom end of the 5% to 9% compounded annual growth rate (CAGR) range for 2020 to 2023. At the same time, Fresenius specifies its Group organic sales growth target to reach the low-end of the targeted 4% to 7% compounded annual growth rate (CAGR) range for 2020 to 2023.
Cost and efficiency program
The Group’s cost and efficiency program is running according to plan and Fresenius confirms its increased savings targets provided in February 2022 of at least €150 million p.a. after tax and minority interest in 2023. For the years thereafter, a further significant increase in sustainable cost savings is expected.
Management Board change at Fresenius Medical Care
Dr. Carla Kriwet will now join Fresenius Medical Care as CEO on October 1, 2022, earlier than previously announced and Rice Powell will step down as CEO effective September 30, 2022.
Preliminary Q2 and H1/22 results2
1Net income attributable to shareholders of Fresenius SE & Co. KGaA
2EBIT and net income before special items
3Excluding Ivenix acquisition
Fresenius Kabi preliminary financial results
Sales in Q2/22 increased by 8% (2% in constant currency) to €1,896 million (Q2/21: €1,755 million). Organic growth was 2%. The positive currency translation effects of 6% in Q2/22 were mainly related to the U.S. dollar and Chinese yuan.
Sales in North America increased by 16% (organic growth: 3%) to €606 million (Q2/21: €522 million), strongly supported by U.S. Dollar-related currency translation effects. Sales in Europe increased by 4% (organic growth: 4%) to €658 million (Q2/21: €634 million). Sales in Asia-Pacific increased by 4% (organic growth: -4%) to €425 million (Q2/21: €409 million). Positive currency translation effects contributed to reported sales growth. Sales in Latin America/Africa increased by 9% (organic growth: 2%) to €207 million (Q2/21: €190 million). Sales for the Biosimilars business were €29 million.
EBIT1 decreased by 9% (-15% in constant currency) to €271 million (Q2/21: €298 million). The EBIT margin1 was 14.3% (Q2/21: 17.0%).
Fresenius Kabi EBIT by region
Fresenius Kabi confirms its FY/22 outlook and expects organic sales3 growth in a low-single-digit percentage range. Constant currency EBIT2,4 is expected to decline in a high-single- to low-double-digit percentage range. The sales and EBIT outlook ranges include expected COVID-19 effects and exclude the effects of the acquisitions Ivenix and mAbxience.
1Before special items
2Excluding Ivenix acquisition
3FY/21 base: €7,193 million
4FY/21 base: €1,153 million, before special items, FY/22 before special items
Fresenius Helios preliminary financial results
Sales increased by 7% (6% in constant currency) to €2,925 million (Q2/21: €2,738 million). Organic growth was 5%. Acquisitions contributed 1% to sales growth.
Sales of Helios Germany increased by 5% (organic growth: 4%) to €1,758 million (Q2/21: €1,675 million). Sales of Helios Spain increased by 8% (7% in constant currency) to €1,101 million (Q2/21: €1,020 million). Organic growth was 6%. Sales of Helios Fertility were €65 million (Q2/21: €42 million).
EBIT1 of Fresenius Helios increased by 2% (1% in constant currency) to €303 million (Q2/21: €298 million) with an EBIT margin1 of 10.4% (Q2/21: 10.9%).
EBIT of Helios Germany increased by 1% to €154 million (Q2/21: €152 million) with an EBIT margin of 8.8% (Q2/21: 9.1%). EBIT of Helios Spain increased by 1% (0% in constant currency) to €148 million (Q2/21: €147 million). The EBIT margin was 13.4% (Q2/21: 14.4%). EBIT1 of Helios Fertility was €7 million with an EBIT1 margin of 10.8% (Q2/21: €5 million).
Fresenius Helios confirms its FY/22 outlook and expects organic sales2 growth in a low-to mid-single-digit percentage range and constant currency EBIT3 growth in a mid-single-digit percentage range. The sales and EBIT outlook ranges include expected COVID-19 effects.
Fresenius Vamed preliminary financial results
Sales increased by 1% (1% in constant currency) to €562 million (Q2/21: €556 million). Organic growth was 1%.
Sales in the service business increased by 6% (6% in constant currency) to €417 million (Q2/21: €392 million). Sales in the project business decreased by 12% (-12% in constant currency) to €145 million (Q2/21: €164 million).
EBIT1 decreased by 31% to €11 million (Q2/21: €16 million) with an EBIT margin1 of 2.0% (Q2/21: 2.9%).
Order intake was €253 million (Q2/21: €713 million). As of June 30, 2022, order backlog was at €3,732 million (December 31, 2021: €3,473 million).
Fresenius Vamed confirms its FY/22 outlook and expects organic sales4 growth in a high-single to low-double-digit percentage range and constant currency EBIT5 to return to absolute pre-COVID-19 levels (FY/19: €134 million). The sales and EBIT outlook ranges include expected COVID-19 effects.
1Before special items
2FY/21 base: €10,891 million
3FY/21 base: €1,127 million, before special items, FY/22 before special items
4FY/21 base: €2,297 million
5FY/21 base: €101 million, before special items; FY/22 before special items
Detailed financial results publication and Conference Call
As part of the publication of the preliminary results for Q2/2022, a conference call will be held on July 28, 2022 at 1:30 p.m. CEDT (7:30 a.m. EDT) replacing the originally planned call from August 2, 2022.
All investors are cordially invited to follow the conference call in a live broadcast over the Internet at www.fresenius.com/investors. Following the call, a replay will be available on our website.
On August 2, 2022, Fresenius will publish detailed Q2/22 and H1/22 financials.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
- Outlook:
- Revised FY 2022 targets: revenue to grow at the low end of the previously guided low to mid-single digit percentage range and net income to decline around a high teens percentage range.
- Despite most burdens assumed to be temporary, 2025 targets withdrawn due to uncertainty of labor and macro-economic inflationary environment
- Unprecedented U.S. labor market situation constraining capacity and accelerating wage inflation
- Worsening macroeconomic environment driving cost inflation and supply chain disruptions
- FME25: Transformation to new operating model and savings generation on track
- Start date for Dr. Carla Kriwet as CEO advanced to October 1, 2022
Helen Giza, Deputy CEO and Chief Financial Officer of Fresenius Medical Care, said: “At the end of the first quarter we assumed extended labor shortages but clearly did not expect such a significant and rapid deterioration. Increased staff shortages, higher staff turnover rates and growing reliance on contract labor continue to increase our cost base, despite support received from the U.S. Provider Relief Fund. At the same time, these factors are constraining our capacity and hence our ability to deliver the volume recovery in Health Care Services that we had assumed for the back half of the year. The already challenging macroeconomic environment has significantly deteriorated – driving non-wage cost inflation and supply chain disruptions. Today we have to assume that these effects will have a very pronounced impact on our business development in the remainder of 2022. Even though most of the current burdens are assumed to be temporary, the uncertainty of these effects is widening the gap to our targets and making a potential catch-up unlikely. As a consequence, we have cut our financial targets for the fiscal year 2022 and feel it prudent to withdraw our 2025 targets. We continue to assess opportunities to accelerate and broaden our FME25 transformation program. We strongly believe our business model and the underlying growth drivers to be intact. Our strategy to drive growth in home dialysis and value-based care is more relevant than ever.”
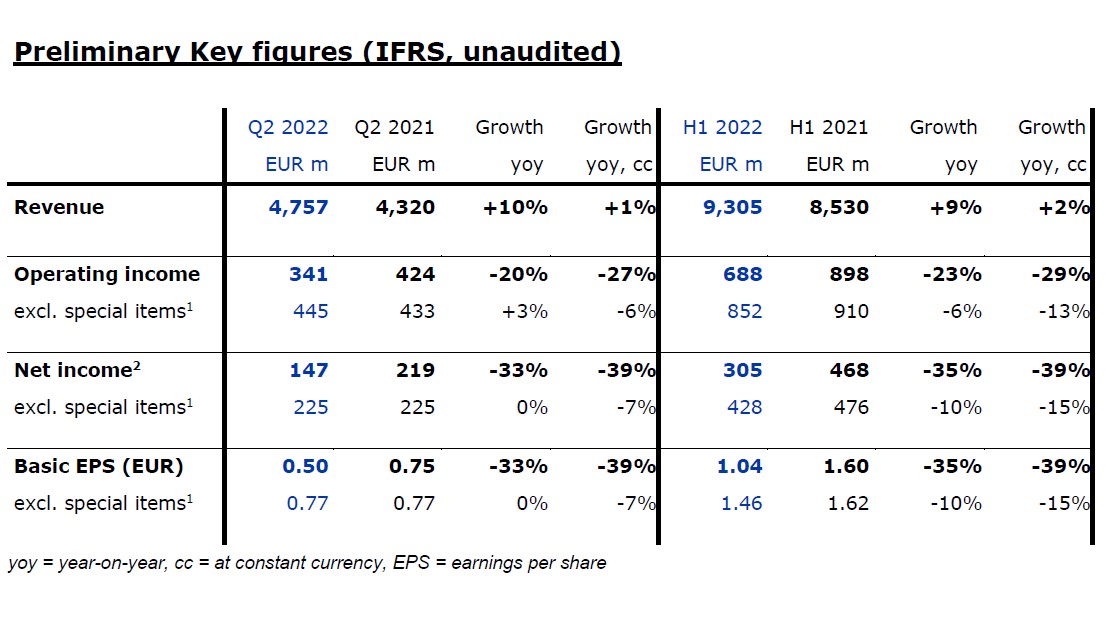
Fresenius Medical Care, the world's leading provider of products and services for individuals with kidney diseases, has announced that revenue and net income2 for the second quarter of 2022 are anticipated to come in below the Company’s expectations. Based on preliminary figures and at constant currency, Fresenius Medical Care expects revenue to increase year-on-year by 1%. For net income excluding special items3 and at constant currency the Company expects a decrease by 7% compared to the previous year’s quarter. Against the background of these preliminary results as well as the significantly changed developments and corresponding materially worsened assumptions for the remainder of the year outlined below, Fresenius Medical Care has cut its financial targets for FY 2022 and withdrawn its 2025 targets.
1 Special items include costs related to the FME25 program, the impact of the War in Ukraine, the impact of hyperinflation in Turkiye, the remeasurement effect on the fair value of the investment in Humacyte, Inc. (Humacyte investment remeasurement) and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance. These items are excluded to ensure comparability of the figures presented with the Company’s financial targets which have been defined excluding special items.
2 Attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
3 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA | 2021 and 2022 figures exclude special items (2021: Costs related to the FME25 program; 2022: Costs related to the FME25 program, impact of the War in Ukraine, impact of Hyperinflation in Turkiye, Humacyte investment remeasurement
Increased headwinds from labor and inflation
The unprecedented U.S. labor market challenges materially worsened in the second quarter. For Fresenius Medical Care, this resulted in meaningfully higher than assumed wage inflation, surcharges, retention payments and additional costs for contract labor to contain the increasing staff shortages.
Despite these additional investments in labor, including application of monies received from the U.S. government's Provider Relief Fund, staff shortages and turnover rates have continued to increase. The Company’s growth in the second quarter was affected by the number of clinics with constrained ability to accept new patients for treatment. Assuming that the unprecedented pressures from the U.S. labor market will persist in the second half of the year, Fresenius Medical Care no longer expects to achieve organic revenue growth in North American Health Care Services in 2022.
The already existing challenging macroeconomic environment has further significantly deteriorated in the second quarter, driving accelerated non-wage cost inflation. This has been exacerbated by the ongoing war in Ukraine and its global economic impact. These effects are expected to persist for the remainder of the year, resulting in higher logistics costs, raw material and energy prices as well as further supply chain disruptions.
Based on preliminary figures, COVID-19-related excess mortality in the second quarter sequentially declined in line with the Company’s projections. However, infection rates remained on a high level resulting in a continued need and costs for isolation clinics and shifts as well as a higher than assumed spend for personal protective equipment.
In the second quarter, revenue in the Healthcare Services business was negatively impacted by meaningful yet unforeseen declines in co-insurance, increases in patient choice of higher deductibles plans, and lower than expected collections in aged accounts receivable.
Revised financial targets for FY 2022
Given the significantly increased headwinds outlined above, Fresenius Medical Care now expects revenue to grow at the low end of the previously guided low to mid-single digit percentage range. For net income2 the Company now expects a decline of around a high teens percentage range compared to the previously guided growth of a low to mid-single digit percentage range. Revenue and net income guidance are both on a constant currency basis and before special items4.
These targets are based on the following operating income relevant assumptions:
- Macro-economic inflation and supply chain costs of around EUR 220 million instead of EUR 50 million previously assumed
- COVID-19: Impact of accumulated excess mortality of around EUR 100 million
- U.S. labor costs expected to be around EUR 100 million, net of support from U.S. Provider Relief Fund, in excess of the 3% base wage inflation assumption
- U.S. ballot initiative expense of EUR 20 to 30 million
- Business growth of EUR 70 million instead of EUR 250 million previously assumed
- Personal protective equipment cost reduction to be around EUR 20 million instead of EUR 50 million previously assumed
- FME25 savings of EUR 40 to 70 million
- Remeasurement effects on the fair value of investments are expected to be volatile but neutral on a full year basis; for guidance relevant comparison, the Humacyte investment remeasurement is treated as special item
- No meaningful further impact from natural gas shortages or suspension of gas supply to affect manufacturing sites
4 These targets are based on the 2021 results excluding the costs related to FME25 of EUR 49 million (for Net Income). They are in constant currency and exclude special items. Special items include further costs related to FME25, the impact of the War in Ukraine, the impact of Hyperinflation in Turkiye, the Humacyte investment remeasurement and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance.
Most of the unprecedented challenges outlined above are assumed to be temporary. However, given the uncertain labor situation and macro-economic inflationary environment and the substantially reduced earnings base compared to 2020, Fresenius Medical Care does not expect today to be able to achieve the meaningfully higher compounded annual average increases that would now be needed to accomplish its 2025 targets. Fresenius Medical Care remains committed to its growth strategy and will consistently pursue the initiatives defined therein.
Preliminary consolidated figures
Revenue increased by 10% to EUR 4,757 million (+1% at constant currency, +0% organic) in the second quarter.
Health Care Services revenue grew by 11% to EUR 3,782 million (+1% at constant currency, +0% organic). Growth at constant currency was mainly driven by contributions from acquisitions.
Health Care Products revenue increased by 6% to EUR 975 million (+1% at constant currency, +1% organic). Constant currency growth was mainly driven by higher sales of in-center disposables, partially offset by lower sales of acute cardiopulmonary products.
In the first half, revenue grew by 9% to EUR 9,305 million (+2% at constant currency, +1% organic). Health Care Services revenue increased by 10% to EUR 7,389 million (+2% at constant currency, +1% organic); Health Care Products revenue grew by 6% to EUR 1,916 million (+2% at constant currency, +2% organic).
Operating income decreased by 20% to EUR 341 million (-27% at constant currency) in the second quarter, resulting in a margin of 7.2% (Q2 2021: 9.8%). Operating income excluding special items, i.e. costs incurred for FME25, the impacts related to the war in Ukraine, the impact of hyperinflation in Turkiye, and the remeasurement effect on the fair value of the investment in Humacyte, Inc. (Humacyte investment remeasurement), increased by 3% to EUR 445 million (-6% at constant currency), resulting in a margin of 9.4% (Q2 2021: 10.0%). At constant currency, the decline was mainly due to higher labor costs as well as inflationary and supply chain cost increases. This was partially offset by Provider Relief Funding received from the U.S. government to compensate for certain COVID-19-related costs.
In the first half, operating income declined by 23% to EUR 688 million (-29% at constant currency), resulting in a margin of 7.4% (H1 2021: 10.5%). Excluding special items, operating income decreased by 6% to EUR 852 million (-13% at constant currency), resulting in a margin of 9.2% (H1 2021: 10.7%).
Net income2 decreased by 33% to EUR 147 million (-39% at constant currency). Excluding special items, net income2 was stable and amounted to EUR 225 million (-7% at constant currency). At constant currency, the decline was mainly due to the mentioned negative effects on operating income. Basic earnings per share (EPS) decreased by 33% to EUR 0.50 (-39% at constant currency). Excluding special items, EPS was stable and amounted to EUR 0.77 (-7% at constant currency).
In the first half, net income2 declined by 35% to EUR 305 million (-39% at constant currency). Excluding special items, net income2 decreased by 10% to EUR 428 million
(-15% at constant currency). EPS decreased by 35% to EUR 1.04 (-39% at constant currency). Excluding special items, EPS declined by 10% to EUR 1.46 (-15% at constant currency).
Regional developments5
5 Based on preliminary figures
In North America, revenue increased by 12% to EUR 3,294 million (-1% at constant currency, -2% organic) in the second quarter. At constant currency, this was mainly due to a decline in organic growth – which was driven by COVID-19 as well as by declines in co-insurance, increases in patient choice of higher deductibles plans, and lower than expected collections in aged accounts receivable in the Health Care Services business – and due to lower sales of in-center disposables, machines for chronic treatment, renal pharmaceuticals and home hemodialysis products. These effects were only partially offset by contributions from acquisitions. In the first half, revenue grew by 10% to
EUR 6,464 million (+0% at constant currency, -1% organic).
Operating income in North America decreased by 14% to EUR 340 million (-24% at constant currency) in the second quarter, resulting in a margin of 10.3% (Q2 2021: 13.5%). At constant currency, the decline in operating income was mainly due to higher labor costs, the Humacyte investment remeasurement, declines in co-insurance, increases in patient choice of higher deductibles plans, and lower than expected collections in aged accounts receivable, the impact of COVID-19, as well as inflationary and supply chain costs. This was partially offset by provider relief funding received from the U.S. government to compensate for certain COVID-19-related costs. In the first half, operating income declined by 19% to EUR 644 million (-26% at constant currency), resulting in a margin of 10.0% (H1 2021: 13.6%).
Revenue in the EMEA region increased by 5% to EUR 727 million in the second quarter (+7% at constant currency, +6% organic). At constant currency, this was mainly due to organic growth in Health Care Services and Health Care Products, both including the effects of hyperinflation in Turkiye. Growth in Health Care Products was driven by higher sales of in-center disposables, machines for chronic treatment and renal pharmaceuticals, partially offset by lower sales of acute cardiopulmonary products. In the first half, revenue grew by 3% to EUR 1,401 million (+5% at constant currency, +4% organic).
Operating income in EMEA decreased by 19% to EUR 60 million (-18% at constant currency) in the second quarter, resulting in a margin of 8.2% (Q2 2021: 10.6%). At constant currency, the decline in operating income was mainly due to inflationary cost increases, the impact of hyperinflation in Turkiye and costs associated with the FME25 program, partially offset by favorable currency transaction effects. In the first half, operating income declined by 21% to EUR 121 million (-18% at constant currency), resulting in a margin of 8.6% (H1 2021: 11.2%).
In Asia-Pacific, revenue increased by 6% to EUR 516 million (+2% at constant currency, +2% organic) in the second quarter. At constant currency, this was mainly driven by organic growth in the Health Care Services business. In the first half, revenue increased by 7% to EUR 1,023 million (+3% at constant currency, +3% organic).
Operating income decreased by 16% to EUR 71 million (-16% at constant currency) in the second quarter, resulting in a margin of 13.8% (Q2 2021: 17.3%). At constant currency, the decline in operating income was mainly due to an unfavorable impact from growth in lower margin businesses and inflationary cost increases. In the first half, operating income was stable and amounted to EUR 170 million (-1% at constant currency), resulting in a margin of 16.6% (H1 2021: 17.7%).
Latin America revenue increased by 21% to EUR 207 million (+17% at constant currency, +18% organic) in the second quarter, mainly driven by organic growth in the Health Care Services business, as well as higher sales of in-center disposables and machines for chronic treatment. In the first half, revenue grew by 18% to EUR 391 million (+16% at constant currency, +17% organic).
Operating income decreased to EUR -6 million in the second quarter, resulting in a margin of -3.0% (Q2 2021: 1.5%). At constant currency, the decline in operating income was mainly due to inflationary cost increases and unfavorable foreign currency transaction effects, partially offset by lower bad debt expense. In the first half, operating income decreased by 46% to EUR 5 million (-71% at constant currency), resulting in a margin of 1.3% (H1 2021: 2.8%).
Patients, clinics and employees5
As of June 30, 2022, Fresenius Medical Care treated 345,687 patients in 4,163 dialysis clinics worldwide and had 123.153 employees (full-time equivalents) globally, compared to 123,538 employees as of June 30, 2021.
FME25 update
With savings of EUR 26 million in the first half of the year, Fresenius Medical Care is on track to achieve its savings target of EUR 40-70 million in 2022 as part of the FME25 transformation program. Key achievements in the first half of the year include the announcement of the first two leadership levels below the Management Board and the corresponding organizational structure as well as the finalization of country governance in line with the future operating model. The Company has also made significant progress in the transformation of global functions such as Finance, Digital Technology & Innovation as well as Procurement. In addition to the ongoing and already identified FME25 measures, Fresenius Medical Care is currently in the process of reviewing potential additional initiatives in both designated operating segments (Care Delivery and Care Enablement) as part of the transformation program.
New CEO start advanced
The start of Dr. Carla Kriwet as CEO of Fresenius Medical Care has been advanced to October 1, 2022. Rice Powell will step down as CEO effective September 30, 2022.
Conference call
Fresenius Medical Care will host a conference call on July 28, 2022 at 9:00 a.m. CEST to discuss the preliminary results for the second quarter and first half of 2022. This conference call will replace the earnings call originally scheduled for August 2, 2022. Details will be available on www.freseniusmedicalcare.com in the “Investors/Publications” section. A replay will be available shortly after the call. The Company will publish its full results for the first quarter and second half of 2022 on August 2, 2022.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to COVID-19, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Implementation of measures as presented herein may be subject to information and consultation procedures with works councils and other employee representative bodies, as per local laws and practice. Consultation procedures may lead to changes on proposed measures.
The information and documents contained on the following pages of this website are for information purposes only. These materials do neither constitute an offer nor an invitation to subscribe to or to purchase securities, nor any investment advice or service, and are not meant to serve as a basis for any kind of obligation, contractual or otherwise. Securities may not be offered or sold in the United States of America (“US”) absent registration under the US Securities Act of 1933, as amended, or an exemption from registration. The securities described on the following pages are not offered for sale in the US or to "US persons" (as defined in Regulation S under the US Securities Act of 1933, as amended).
THE FOLLOWING INFORMATION AND DOCUMENTS ARE NOT DIRECTED AT AND ARE NOT INTENDED FOR USE BY (I) PERSONS WHO ARE RESIDENTS OF OR LOCATED IN THE US, CANADA, JAPAN OR AUSTRALIA OR WHO ARE US PERSONS (AS DEFINED IN REGULATION S UNDER THE US SECURITIES ACT OF 1933, AS AMENDED), OR (II) PERSONS IN ANY OTHER JURISDICTION WHERE THE COMMUNICATION OR RECEIPT OF SUCH INFORMATION IS RESTRICTED IN SUCH A WAY THAT PROVIDES THAT SUCH PERSONS SHALL NOT RECEIVE IT. SUCH PERSONS, OR PERSONS ACTING FOR THE BENEFIT OF ANY SUCH PERSONS, ARE NOT PERMITTED TO VISIT THE FOLLOWING PAGES OF THE WEBSITE.
To visit the following parts of this website you must confirm that
(i) you are not a resident of the United States of America, Canada, Japan or Australia or a "US person" (as defined in Regulation S under the US Securities Act of 1933, as amended),
(ii) you are not a person to whom the communication of the information contained on the website is restricted,
(iii) you will not distribute any of the information and documents contained thereon to any such person, and
(iv) you are not acting for the benefit of any such person.
By clicking on the "Accept" button below, you will be deemed to have made this confirmation.
NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES OF AMERICA, AUSTRALIA, CANADA OR JAPAN.
Fresenius today successfully placed bonds with an aggregate volume of €1.3 billion across two tranches:
- €750 million bonds with a maturity in May 2025 and an annual coupon of 1.875% and
- €550 million bonds with a maturity in May 2030 and an annual coupon of 2.875%.
The proceeds will be used for general corporate purposes, including refinancing of existing financial liabilities.
The bonds were drawn under the Fresenius Debt Issuance Program (DIP) and issued by Fresenius SE & Co KGaA. Fresenius has applied to the Luxembourg Stock Exchange to admit the bonds to trading on its regulated market.
The envisaged settlement date is May 24, 2022.
This announcement does not contain or constitute an offer of, or the solicitation of an offer to buy or subscribe for, securities to any person in Australia, Canada, Japan, or the United States of America (the “United States”) or in any jurisdiction to whom or in which such offer or solicitation is unlawful. The securities referred to herein may not be offered or sold in the United States or to, or for the account or benefit of, U.S. persons, absent registration under the U.S. Securities Act of 1933, as amended (the “Securities Act”) except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act. Subject to certain exceptions, the securities referred to herein may not be offered or sold in Australia, Canada or Japan or to, or for the account or benefit of, any national, resident or citizen of Australia, Canada or Japan. The offer and sale of the securities referred to herein has not been and will not be registered under the Securities Act or under the applicable securities laws of Australia, Canada or Japan. There will be no public offer of the securities in the United States.
This announcement is a general information and not a prospectus. Investors should not purchase or subscribe for any securities referred to in this announcement except on the basis of information in the prospectus to be issued by the company in connection with the offering of such securities. Copies of the prospectus will, following publication, be available free of charge from Fresenius SE & Co. KGaA at Else-Kröner Strasse 1, 61352 Bad Homburg, Germany.
This announcement has been prepared on the basis that any offer of securities in any Member State of the European Economic Area (EEA) will be made pursuant to the prospectus prepared by Fresenius SE & Co. KGaA, Fresenius Finance Ireland Public Limited Company and Fresenius Finance Ireland II Public Limited Company in combination with the relevant final terms relating to such securities or pursuant to an exemption under Regulation (EU) 1129/2017 (the Prospectus Regulation) from the requirement to publish a prospectus for offers of securities. Neither Fresenius SE & Co. KGaA, Fresenius Finance Ireland Public Limited Company nor Fresenius Finance Ireland II Public Limited Company have authorized, nor do they authorize, the making of any offer of securities in circumstances in which an obligation arises for Fresenius SE & Co. KGaA, Fresenius Finance Ireland Public Limited Company and Fresenius Finance Ireland II Public Limited Company or any other person to publish or supplement a prospectus for such offer.
This announcement is directed at and/or for distribution in the United Kingdom only to (i) persons who have professional experience in matters relating to investments falling within article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (ii) high net worth entities falling within article 49(2)(a) to (d) of the Order (all such persons are referred to herein as “relevant persons”). This announcement is directed only at relevant persons. Any person who is not a relevant person should not act or rely on this announcement or any of its contents. Any investment or investment activity to which this announcement relates is available only to relevant persons and will be engaged in only with relevant persons.
This announcement contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts. Neither Fresenius SE & Co. KGaA, Fresenius Finance Ireland Public Limited Company nor Fresenius Finance Ireland II Public Limited Company undertake any responsibility to update the forward-looking statements in this announcement.
Despite ongoing burdens from the pandemic, the war in Ukraine and other external factors, the global healthcare group Fresenius remains on course for growth. This was confirmed by Stephan Sturm, CEO of Fresenius, at today’s Annual General Meeting: “We have some challenges to overcome, no question! But our path is clear. We have set strategic guidelines to achieve sustainable and accelerating profitable growth. Our goal is, and remains, to create value and benefit for all our stakeholders. By doing what we have done best for 110 years: providing high-quality medicine at affordable prices, tailored to the needs of more and more people around the world who need medical care.”
Sturm said the company’s growth strategy, first set out last year and recently defined in more detail, will make a major contribution here. It includes a company-wide cost-cutting and efficiency program, the accessing of new sources of capital, and prioritizing the distribution of capital among the business segments. “We see continued excellent growth opportunities for all four business segments. Our decisions will enable the accelerated growth of each individual business segment, and thereby also accelerate growth for the entire Group,“ Sturm said. “We want to move Fresenius ahead at speed. And we want a measured and well-managed transformation of our company. Fresenius remains a diversified healthcare group, with a sharper profile, active in wide-ranging and very exciting areas of medicine.”
In his speech, Sturm sharply condemned Russia’s attack on Ukraine: “As a healthcare company, Fresenius fights to save lives: Putin’s army fights to destroy an entire country, with a contempt for people and a brutality that appalls me.” At the same time, the Fresenius CEO explained why the company is not pulling out of Russia: “Because that is also part of our responsibility as a healthcare company. Many of our products and services are essential for life. Our patients depend on them – also in Russia. We cannot simply refuse them life-saving treatments and coldly stand aside and let them die.” Sturm stressed that Fresenius is not making any money in Russia and will not in the foreseeable future, and has put all investments in the country on ice.
Shareholders approved with a large majority of 99.87 percent the 29th consecutive dividend increase proposed by the General Partner and the Supervisory Board. The dividend was raised by 5 percent, to €0.92 per share.
Fresenius is offering a scrip dividend for the first time, thereby giving shareholders the option to receive their dividend (except for the tax portion of the dividend) in the form of new Fresenius shares. The Else Kröner-Fresenius-Foundation has informed Fresenius that it intends to fully participate in the scrip dividend.
The Annual General Meeting elected Susanne Zeidler and Dr. Christoph Zindel to the Supervisory Board of Fresenius SE & Co. KGaA with large majorities. The new elections were made necessary by the departures of Hauke Stars and Klaus-Peter Müller.
The shareholders also approved with a large majority, of 90.47 percent, the Compensation Report for the 2021 business year.
With a majority of 89.09 percent, the shareholders approved a new Authorized Capital I in the amount of €125 million. New authorizations to issue convertible bonds, to repurchase own shares, and to use equity derivatives for repurchasing own shares were also approved.
Shareholder majorities of 99.02 and 92.57 percent, respectively, approved the actions of the Management and Supervisory Boards in 2021.
At the Annual General Meeting of Fresenius SE & Co. KGaA, 73.08 percent of the subscribed capital was represented. Due to the pandemic, the meeting was held exclusively over the Internet in order to protect the health of all participants.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, the availability of financing and unforeseen impacts of international conflicts.
Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, is returning to a growth path after the significant burdens of the COVID-19 pandemic. At today’s Annual General Meeting, Chief Executive Officer Rice Powell confirmed the company’s outlook for 2022: Revenue and net income are expected to grow at low to mid-single digit percentage rates.
“We obviously had to overcome some challenges. Despite the unprecedented effects of COVID-19, we believe that the fundamental drivers of our business and growth remain unchanged,” Powell said in his speech to shareholders. “People across the world are living longer. As the population continues to age, we estimate that more than six million people will require dialysis by 2030 – a 460 percent increase compared to 2000. Our Strategy 2025 puts the company in a position to leverage the opportunities these developments bring and guarantee sustainable profitable growth in the future.”
Strategy 2025 aims at Fresenius Medical Care’s expansion along the renal care continuum. Powell said value-based care programs, in particular, are an important step toward success: “They allow us to keep costs affordable for payors in private and public settings, while improving our patients’ quality of life with ever better products and services.” In addition, the rollout of the company’s new operating model is on track: The FME25 transformation program is set to achieve its first sustained savings this year. After a one-time investment of EUR 450 to 500 million, most cost-saving measures will be implemented by 2024, reducing the annual cost base by EUR 500 million by the end of 2025.
It was the last Annual General Meeting for Rice Powell as CEO of Fresenius Medical Care. As the company announced earlier this month, he will enter retirement when his contract expires on December 31, 2022 and be succeeded as CEO by Dr. Carla Kriwet, who introduced herself personally to the shareholders at today's Annual General Meeting.
A large shareholder majority of 99.49 percent approved the company’s 25th consecutive dividend increase. The dividend will be raised from €1.34 to €1.35 per share.
By another large majority of 94.87 percent, the shareholders approved the compensation report for fiscal year 2021.
Shareholder majorities of 97.65 and 91.69 percent, respectively, approved the actions of the General Partner and the Supervisory Board in 2021.
At the Annual General Meeting, 80.76 percent of the registered capital was represented. Because of the pandemic, the meeting again was held as a purely virtual event in order to protect the health of everyone involved.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Implementation of measures as presented herein may be subject to information & consultation procedures with works councils and other employee representative bodies, as per local laws and practice. Consultation procedures may lead to changes on proposed measures.
- Further earnings growth in 2022 expected despite ongoing COVID-19 effects, and cost inflation impact
- Accelerated execution of cost and efficiency program leading to earlier and significantly higher savings
- Medium-term growth targets confirmed and specified
- 29th consecutive dividend increase – scrip dividend proposed
- Fresenius to be climate neutral by 2040
If no timeframe is specified, information refers to Q4/2021.
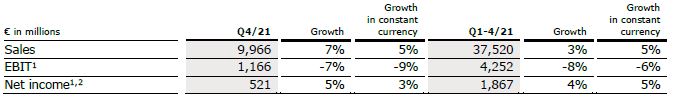
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Stephan Sturm, CEO of Fresenius, said: “Our mission is to protect people’s health. Fulfilling that mission has rarely been as difficult as during this pandemic. But we have done our part and have lived up to our responsibility. In business terms, too, 2021 was challenging yet successful: We delivered a strong final quarter and fully met our targets for the year. In 2022 we expect continued profitable growth, despite rising inflation and the ongoing burdens caused by the pandemic. In our cost and efficiency program, we have made faster than expected progress. This is an important factor enabling us to confirm the medium-term targets we set well before the pandemic, giving us all the more reason to look ahead with optimism.”
Path to accelerated growth
Fresenius has defined a strategic path to pursue accelerated profitable growth and hence to sustainably strengthen the Group and each of its business segments by tapping new sources of capital and prioritizing segment capital allocation. All our stakeholders continue to benefit from the advantages of the Group’s current structure, which offers stability through diversification as well as efficiency through economies of scale, access to attractive debt financing and tax savings.
All of Fresenius’ business segments have excellent market positions and ample meaningful growth opportunities. Properly balancing the objectives of all our stakeholder groups requires an even more targeted approach to capital allocation. While Fresenius continues to believe in the virtues of vertical integration, the Company is keen to gradually re-balance the relative weights of its products and service businesses.
Primarily based on its superior profitability and excellent growth prospects, Fresenius Kabi is defined as top priority. With respect to Fresenius Medical Care, which has been particularly hard hit by the pandemic, the transformation program FME25 is expected to result in ever improving profitability and accelerated growth, driving improved valuation for Fresenius’ controlling stake. For Fresenius Helios and Fresenius Vamed, smaller inorganic growth opportunities will continue to be financed from Fresenius Group funds. For larger growth opportunities, Fresenius is open to value-enhancing external equity investments at the level of these business segments. An equity increase on Group level would then be redundant and is hence not foreseen.
By setting this course, Fresenius will accelerate the growth of each of our business segments for the benefit of all stakeholders.
“We are moving Fresenius ahead at speed, with a measured and well-managed transformation of our company. All our business segments have strong market positions, and great growth potential. We intend to harness this potential – guided by clear strategic priorities that will combine additional sources of more dynamic growth with the advantages of a broad business structure. Fresenius remains a diversified healthcare group, with a sharper profile, that will be active in wide-ranging and very exciting areas of medicine,” said Stephan Sturm, CEO of Fresenius.
FY/22 Group guidance
For FY/22, Fresenius projects sales growth1 in a mid-single-digit percentage range in constant currency. Net income2,3 is expected to grow in a low-single-digit percentage range in constant currency. Implicitly, net income2 for the Group excluding Fresenius Medical Care is expected to grow in a low-single-digit percentage range in constant currency.
Without further acquisitions, Fresenius projects an improvement of the net debt/EBITDA4 ratio (December 31, 2021: 3.51x5) into the self-imposed target corridor of 3.0x to 3.5x by the end of 2022.
Assumptions for guidance FY/22
COVID-19 will continue to impact Fresenius’ operations in 2022. The extent of the impact on the Group is to a large degree dependent on the vaccination coverage in Fresenius’ relevant markets and the potential evolution of new virus mutants.
Fresenius closely monitors the development of the COVID-19 pandemic and the associated various containment measures enacted in the Company’s relevant markets. Fresenius expects COVID-19 case numbers to decline from spring 2022 onwards and consequently the number of elective treatments and staff availability to improve. A possible significant deterioration of the situation associated with further containment measures that could have a significant and direct impact on the health care sector without any appropriate compensation is not reflected in the Group’s FY/22 guidance.
1 FY/21 base: €37,520 million
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 FY/21 base: €1,867 million; before special items; FY/22: before special items
4 At LTM average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures; excluding further potential acquisitions; before special items; including lease liabilities
5 At LTM average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures; before special items; including lease liabilities
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Headwinds from cost inflation are reflected. However, Fresenius expects no significant acceleration of inflation effects and supply chain challenges versus the current environment. The Management Board assumes an unchanged corporate tax rate in the United States.
Furthermore, the assumptions for Fresenius Medical Care's FY/22 guidance are also fully applicable to Fresenius Group's FY/22 guidance.
All of these assumptions are subject to considerable uncertainty.
Cost and efficiency program leading to significantly higher savings
Fresenius has successfully completed the first phase of its cost and efficiency program aiming to further safeguard the Group’s medium-term targets and to sustainably enhance profitability. This has led to initial cost savings of ~€20 million and one-time expenses of ~€80 million in 2021. Given the good progress, especially driven by the accelerated implementation of initiatives, Fresenius significantly increases its savings target and now expects cost savings of at least €150 million p.a. after tax and minority interest in 2023. Initially, more than €100 million p.a. after tax and minority interest were projected. For the years thereafter, a further significant increase in sustainable cost savings is expected. The savings will be achieved by all four business segments and the corporate center.
Fresenius anticipates that achieving these sustainable efficiency improvements will require up-front expenses of more than €200 million in 2022 and further expenses of around €100 million in 2023, in each case after taxes and minority interest. No further significant expenses are expected thereafter. In line with previous practice, these expenses are classified as special items.
Growth targets for 2020 – 2023 confirmed and specified
Based on the anticipated positive contributions from the cost and efficiency program as well as the attractive growth opportunities across all business segments, Fresenius expects Group earnings growth to meaningfully accelerate until 2023. The company hence confirms its medium-term targets set in 2019 despite the ongoing challenges posed by COVID-19. At the same time, Fresenius specifies its expectations and now anticipates Group organic sales growth to reach the bottom to middle of the targeted 4% to 7% compounded annual growth rate (CAGR) and Group organic net income1,2 growth to be at the bottom end of the 5% to 9% CAGR during 2020 to 2023. Due to the COVID-19 pandemic, Fresenius now expects small and medium-sized acquisitions to contribute an incremental CAGR of less than 1% to both sales and net income growth.
29th consecutive dividend increase proposed
Consistent with Fresenius’ stated policy, the Management Board of Fresenius will propose to the Supervisory Board a dividend increase of 5% to €0.92 per share for FY/21 (FY/20: €0.88). Provided the proposal is approved by the Supervisory Board and the Annual General Meeting, this will be the 29th consecutive dividend increase.
The Management Board will propose a scrip dividend to the Supervisory Board, thereby giving shareholders the option to receive their dividend (except for the tax portion of the dividend) in the form of new Fresenius shares. The Else Kröner-Fresenius-Foundation has informed Fresenius that it intends to fully participate in the scrip dividend.
Fresenius to be climate neutral by 2040
Fresenius has set a climate target for the Group complementing its existing sustainability targets and programs. The company aims to be climate neutral by 2040 and to reduce 50% of absolute scope 1 and scope 2 emissions by 2030 compared to 2020 levels. Fresenius will continuously assess scope 3 emission impacts for inclusion in targets. Further information at https://www.fresenius.com/sustainability and in today’s separate press release at https://www.fresenius.com/news.
1 Net income attributable to shareholders of Fresenius SE & Co. KGaA
2 Before special items
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
5% sales growth in constant currency
Group sales increased by 7% (5% in constant currency) to €9,966 million (Q4/20: €9,304 million). Organic growth was 4%. Acquisitions/divestitures contributed net 1% to sales growth. Currency translation increased sales growth by 2%. Excluding estimated COVID-19 effects1, Group sales growth would have been 5% to 6% in constant currency.
In FY/21, Group sales increased by 3% (5% in constant currency) to €37,520 million (FY/20: €36,277 million). Organic growth was 4%. Acquisitions/divestitures contributed net 1% to sales growth. Currency translation reduced sales growth by 2%. Excluding estimated COVID-19 effects1, Group sales growth would have been 5% to 6% in constant currency.
5% net income2,3 growth in constant currency
Group EBITDA before special items decreased by 2% (-5% in constant currency) to €1,846 million (Q4/202: €1,886 million). Reported Group EBITDA was €1,868 million (Q4/20: €1,854 million).
In FY/21, Group EBITDA before special items decreased by 4% (-2% in constant currency) to €6,854 million (FY/202: €7,132 million). Reported Group EBITDA was €6,825 million (FY/20: €7,100 million).
Group EBIT before special items decreased by 7% (-9% in constant currency) to €1,166 million (Q4/202: €1,251 million). The decrease is primarily due to COVID-19 related headwinds at Fresenius Medical Care. The EBIT margin before special items was 11.7% (Q4/202: 13.4%). Reported Group EBIT was €1,123 million (Q4/20: €1,024 million).
In FY/21, Group EBIT before special items decreased by 8% (-6% in constant currency) to €4,252 million (FY/202: €4,612 million). The decrease is primarily due to COVID-19 related headwinds at Fresenius Medical Care. The EBIT margin before special items was 11.3% (FY/202: 12.7%). Reported Group EBIT was €4,158 million (FY/20: €4,385 million).
1 For estimated COVID-19 effects in Q4/21 and FY/21 please see table on page 19 in the PDF.
2 Before special items
3 Net income attributable to shareholders of Fresenius SE & Co. KGaA
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Group net interest before special items improved to -€120 million (Q4/202: -€159 million) mainly due to successful refinancing activities. Reported Group net interest improved to -€122 million (Q4/20: -€156 million).
In FY/21, Group net interest before special items improved to -€504 million (FY/201: - €654 million) while reported Group net interest improved to -€506 million (FY/20: -€659 million).
The Group tax rate before special items was 23.1% (Q4/201: 24.1%) while the reported Group tax rate was 24.2% (Q4/20: 29.4%). In FY/21, the Group tax rate before special items was 22.6% (FY/201: 23.1%) while the reported Group tax rate was 22.8% (FY/20: 24.2%).
Noncontrolling interests before special items were €283 million (Q4/201: €335 million) of which 90% were attributable to the noncontrolling interests in Fresenius Medical Care. Reported noncontrolling interests were €260 million (Q4/20 reported: €203 million).
In FY/21, noncontrolling interests before special items were €1,033 million (FY/201: €1,248 million) of which 91% were attributable to the noncontrolling interests in Fresenius Medical Care. Reported noncontrolling interests were €1,001 million (FY/20 reported: €1,116 million).
Group net income2 before special items increased by 5% (3% in constant currency) to €521 million (Q4/201: €494 million). The increase is driven by the strong development of Fresenius Kabi’s Emerging Market business, a good performance at Helios Germany, an excellent finish to the year by Fresenius Vamed and the favorable net interest development. Excluding estimated COVID-19 effects3, Group net income2 before special items would have grown 3% to 7% in constant currency. Reported Group net income2 increased to €499 million (Q4/20: €410 million).
In FY/21, Group net income2 before special items increased by 4% (5% in constant currency) to €1,867 million (FY/201: €1,796 million). Excluding estimated COVID-19 effects3, Group net income2 before special items would have grown 6% to 10% in constant currency. Reported Group net income2 increased to €1,818 million (FY/20: €1,707 million).
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 For estimated COVID-19 effects in Q4/21 and FY/21 please see table on page 19 in the PDF.
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Earnings per share1 before special items increased by 5% (2% in constant currency) to €0.94 (Q4/202: €0.88). Reported earnings per share1 were €0.90 (Q4/20: €0.73). In FY/21, earnings per share1 before special items increased by 4% (5% in constant currency) to €3.35 (FY/202: €3.22). Reported earnings per share1 were €3.26 (FY/20: €3.06).
Continued investment in growth
Spending on property, plant and equipment was €690 million corresponding to 7% of sales (Q4/20: €856 million; 9% of sales). These investments served primarily for the modernization and expansion of dialysis clinics, production facilities as well as hospitals and day clinics. In FY/21, spending on property, plant and equipment was €2,032 million corresponding to 5% of sales (FY/20: €2,398 million; 7% of sales).
Total acquisition spending was €278 million (Q4/20: €251 million). In FY/21, total acquisition spending was €1,085 million (FY/20: €902 million) mainly for the acquisition of the Eugin Group at Fresenius Helios which has been consolidated since April 1, 2021, and the acquisition of dialysis clinics at Fresenius Medical Care.
Strong cash flow development in Q4/21
Group operating cash flow increased by 26% to €1,749 million (Q4/20: €1,390 million) with an improved margin of 17.5% (Q4/20: 14.9%) mainly due to stringent working capital management. The good operating performance at Helios Spain, Fresenius Vamed and Fresenius Kabi also contributed to the positive development. Free cash flow before acquisitions and dividends increased to €1,075 million (Q4/20: €590 million). Free cash flow after acquisitions and dividends increased to €841 million (Q4/20: €329 million).
In FY/21, Group operating cash flow decreased to €5,078 million (FY/20: €6,549 million) with a margin of 13.5% (FY/20: 18.1%) mainly due to the U.S. government’s advanced payments received in 2020 and the partial recoupment of these payments in 2021 at Fresenius Medical Care.
Free cash flow before acquisitions and dividends decreased to €3,061 million (FY/20: €4,183 million). Free cash flow after acquisitions and dividends decreased to €1,193 million (FY/20: €2,478 million).
1 Net income attributable to shareholders of Fresenius SE & Co. KGaA
2 Before special items
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Solid balance sheet structure
Group total assets increased by 8% (4% in constant currency) to €71,962 million (Dec. 31, 2020: €66,646 million). The increase is mainly due to currency translation effects, acquisitions as well as the expansion of business activities. Current assets increased by 11% (8% in constant currency) to €17,461 million (Dec. 31, 2020: €15,772 million) driven by the increase of cash and cash equivalents, trade accounts receivables and inventories. Non-current assets increased by 7% (3% in constant currency) to €54,501 million (Dec. 31, 2020: €50,874 million).
Total shareholders’ equity increased by 13% (7% in constant currency) to €29,288 million (Dec. 31, 2020: €26,023 million). The increase is due to currency translation effects as well as the good net income development. The equity ratio was 40.7% (Dec. 31, 2020: 39.0%).
Group debt increased by 5% (2% in constant currency) to €27,155 million (Dec. 31, 2020: € 25,913 million). Group net debt increased by 1% (-1% in constant currency) to € 24,391 million (Dec. 31, 2020: € 24,076 million).
As of December 31, 2021, the net debt/EBITDA ratio increased to 3.51x1,2 (Dec. 31, 2020: 3.44x1,2) driven by COVID-19 effects weighing on EBITDA.
1 At LTM average exchange rates for both net debt and EBITDA; pro forma closed acquisitions/divestitures; including lease liabilities
2 Before special items
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Increased number of employees
As of December 31, 2021, the Fresenius Group had 316,078 employees worldwide (September 30, 2021: 314,852).
Business Segments
Fresenius Medical Care (Financial data according to Fresenius Medical Care press release)
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases. As of December 31, 2021, Fresenius Medical Care was treating 345,425 patients in 4,171 dialysis clinics. Along with its core business, the Renal Care Continuum, the company focuses on expanding in complementary areas and in the field of critical care.
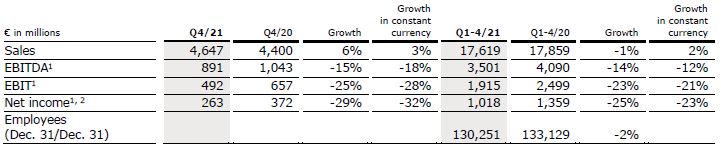
- Business development significantly impacted by COVID-19 in 2021, effects are expected to continue into 2022
- Decline in excess mortality in the fourth quarter
- Return to earnings growth in 2022 targeted
Sales increased by 6% (3% in constant currency) to €4,647 million (Q4/20: €4,400 million). Currency translation increased sales growth by 3%. Organic growth was 2%. Acquisitions/divestitures contributed net 1% to sales growth.
In FY/21, sales decreased by 1% (increased by 2% in constant currency) to €17,619 million (FY/20: €17,859 million). Currency translation decreased sales growth by 3%. Organic growth was 1%. Acquisitions/divestitures contributed net 1% to sales growth.
EBIT decreased by 3% (-7% in constant currency) to €449 million (Q4/20: €462 million) resulting in a margin of 9.7% (Q4/20: 10.5%). EBIT before special items decreased by 25% (-28% in constant currency) to €492 million (Q4/20: €657 million), resulting in a margin of 10.6% (Q4/20: 14.9%). The decline was mainly due to a remeasurement effect on the fair value of investments, higher labor cost, the adverse COVID-19-related net effects and inflationary materials cost increases. These effects were only slightly mitigated by an improved U.S. payor mix, in particular due to an increased number of patients with Medicare Advantage coverage.
1 Before special items
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
In FY/21, EBIT decreased by 20% (-17% in constant currency) to €1,852 million (FY/20: €2,304 million) resulting in a margin of 10.5% (FY/20: 12.9%). EBIT before special items decreased by 23% (-21% in constant currency) to €1,915 million (FY/20: €2,499 million), resulting in a margin of 10.9% (FY/20: 14.0%).
Net income1 increased by 29% (23% in constant currency) to €229 million (Q4/20: €177 million). Net income1 before special items decreased by 29% (-32% in constant currency) to €263 million (Q4/20: €372 million) mainly due to the mentioned negative effects on operating income. In FY/21, net income1 decreased by 17% (-14% in constant currency) to €969 million (FY/20: €1,164 million). Net income1 before special items decreased by 25% (-23% in constant currency) to €1,018 million (FY/20: €1,359 million).
Operating cash flow was €669 million (Q4/20: €584 million) with a margin of 14.4% (Q4/20: 13.3%). The increase was mainly due to improved working capital including contributions from FME25 and U.S. federal relief funding, partially offset by continued recoupment of the U.S. government’s payments received in 2020 under the CARES Act and lower tax payments related to COVID-19 reliefs in the prior year. In FY/21, operating cash flow was €2,489 million (FY/20: €4,233 million) with a margin of 14.1% (FY/20: 23.7%).
For FY/22, Fresenius Medical Care expects revenue2 and net income1,3 to grow at low- to mid-single-digit percentage rates in constant currency4. For the underlying assumptions please see Fresenius Medical Care’s press release at http://www.freseniusmedicalcare.com.
For further information, please see Fresenius Medical Care’s press release at http://www.freseniusmedicalcare.com.
1 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
2 FY/21 base: €17,619 million
3 FY/21 base: €1,018 million, before special items; FY/22 before special items
4 These targets are based on the 2021 results excluding the costs related to FME25 of €49 million (for net income). They are based on the outlined assumptions (http://www.freseniusmedicalcare.com), in constant currency and exclude special items. Special items include further costs related to FME25 and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance.
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Fresenius Kabi
Fresenius Kabi offers intravenously administered generic drugs, clinical nutrition and infusion therapies for seriously and chronically ill patients in the hospital and outpatient environments. The company is also a leading supplier of medical devices and transfusion technology products. In the biosimilars business, Fresenius Kabi develops products with a focus on oncology and autoimmune diseases.
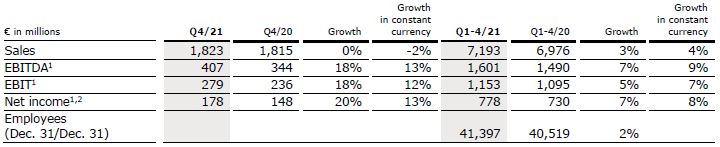
- Good performance in Q4 supported by COVID-driven demand, not expected to continue through 2022
- North America with positive organic sales and EBIT growth despite supply chain challenges
- Asia-Pacific with anticipated organic sales decline due to price effects in China post successful participation in NVBP tenders
- Separate reporting of Biosimilars sales starting Q1/22
Sales remained on previous year’s level (decreased by -2% in constant currency) at €1,823 million (Q4/20: €1,815 million). Organic growth was -1%. Divestitures reduced sales growth by 1%. Positive currency translation effects (2%) were mainly related to the appreciation of the U.S. dollar and the Chinese yuan against the Euro.
In FY/21, sales increased by 3% (4% in constant currency) to €7,193 million (FY/20: €6,976 million). Organic growth was 4%. Negative currency translation effects of 1% were mainly related to the weakness of the U.S. dollar.
Sales in North America increased by 7% (organic growth: 2%) to €589 million (Q4/20: €549 million) driven by COVID-19 related extra demand. In FY/21, sales in North America decreased by 5% (organic growth: -2%) to €2,258 million (FY/20: €2,376 million).
Sales in Europe decreased by 2% (organic growth: 0%) to €664 million (Q4/20: €680 million) mainly due to the high prior-year base. In FY/21, sales in Europe increased by 3% (organic growth: 3%) to €2,544 million (FY/20: €2,458 million).
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Sales in Asia-Pacific decreased by 8% (organic growth: -13%) to €395 million (Q4/20: €428 million) due to the anticipated negative price effects from successful participation in NVBP (National Volume-Based Purchasing) tenders as well as the exceptionally high prior-year base. In FY/21, sales in Asia-Pacific increased by 10% (organic growth: 8%) to €1,643 million (FY/20: €1,497 million).
Sales in Latin America/Africa increased by 11% (organic growth: 12%) to €175 million (Q4/20: €158 million) due to ongoing COVID-19 related extra demand. In FY/21, sales in Latin America/Africa increased by 16% (organic growth: 23%) to €748 million (FY/20: €645 million).
EBIT before special items increased by 18% (12% in constant currency) to €279 million (Q4/201: €236 million) with a margin of 15.3% (Q4/201:13.0%). The excellent performance is primarily due to COVID-19 related extra demand, and cost savings in the Asia-Pacific region, mainly in China. The ongoing competitive situation, supply chain challenges, the flow-through effects of tenders in China were headwinds. There were broadly offsetting one time effects across the regions. In FY/21, EBIT before special items increased by 5% (7% in constant currency) to €1,153 million (FY/201: €1,095 million) with a margin of 16.0% (FY/201: 15.7%).
Net income1,2 increased by 20% (13% in constant currency) to €178 million (Q4/201: €148 million). In FY/21, net income1,2 increased by 7% (8% in constant currency) to €778 million (FY/201: €730 million).
Operating cash flow increased by 9% to €335 million (Q4/20: €307 million) with a margin of 18.4% (Q4/20: 16.9%) mainly due to a healthy operational performance. In FY/21, operating cash flow increased by 5% to €1,203 million (FY/20: €1,143 million) with a margin of 16.7% (FY/20: 16.4%).
For FY/22, Fresenius Kabi expects organic sales3 growth in a low-single-digit percentage range. Constant currency EBIT4 is expected to decline in a high-single- to low-double-digit percentage range. Both sales and EBIT outlook include expected COVID-19 effects.
Starting Q1/22, the sales of the Biosimilars business will be reported on a quarterly basis.
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 FY/21 base: €7,193 million
4 FY/21 base: €1,153 million, before special items, FY/22 before special items
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Fresenius Helios
Fresenius Helios is Europe's leading private hospital operator. The company comprises Helios Germany and Helios Spain (Quirónsalud) and the Eugin Group. Helios Germany operates 90 hospitals, ~130 outpatient centers and 6 prevention centers. Quirónsalud operates 49 hospitals in Spain as well as 88 outpatient centers and ~300 occupational risk prevention centers. In addition, the company is active in Latin America with 7 hospitals and as a provider of medical diagnostics.
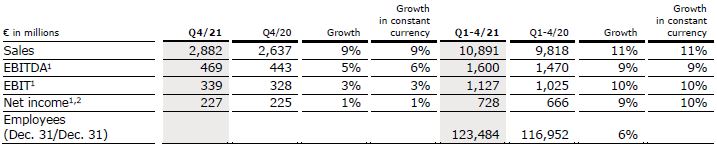
- Sales growth at Helios Germany driven by increasing admissions and acquisitions
- Helios Spain with strong organic sales growth; EBIT growth influenced by exceptionally high prior-year base
- Separate reporting of Fertility Services starting Q1/22
Sales increased by 9% (9% in constant currency) to €2,882 million (Q4/20: €2,637 million). Organic growth was 5%. Acquisitions contributed 4% to sales growth. In FY/21, sales increased by 11% (11% in constant currency) to €10,891 million (FY/20: €9,818 million). Organic growth was 7%. Acquisitions contributed 4% to sales growth.
Sales of Helios Germany increased by 7% (organic growth: 4%) to €1,745 million (Q4/20: €1,637 million) primarily driven by increasing admissions. Acquisitions contributed 3% to sales growth. In FY/21, sales of Helios Germany increased by 6% (organic growth: 2%) to €6,733 million (FY/20: €6,340 million). Acquisitions contributed 4% to sales growth.
Sales of Helios Spain increased by 9% (9% in constant currency) to €1,084 million (Q4/20: €999 million). Organic growth of 9% was driven by the continuous high level of treatment activity and a consistently high level of demand for the occupational risk prevention services as well as good contributions from Latin America. In FY/21, sales of Helios Spain increased by 16% (17% in constant currency) to €4,021 million (FY/20: €3,475 million). Organic growth was 15%. Acquisitions contributed 2% to sales growth.
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
EBIT1 of Fresenius Helios increased by 3% (3% in constant currency) to €339 million (Q4/20: €328 million) with a margin1 of 11.8% (Q4/20: 12.4%). In FY/21, EBIT1 of Fresenius Helios increased by 10% (10% in constant currency) to €1,127 million (FY/20: €1,025 million) with a margin1 of 10.3% (FY/20: 10.4%).
EBIT1 of Helios Germany increased by 9% to €171 million (Q4/20: €157 million) with a margin1 of 9.8% (Q4/20: 9.6%) driven by the positive business development as well as the compensation for COVID-19 related revenue shortfalls. In FY/21, EBIT1 of Helios Germany increased by 2% to €613 million (FY/20: €602 million) with a margin1 of 9.1% (FY/20: 9.5%).
EBIT1 of Helios Spain increased by 2% (3% in constant currency) to €162 million (Q4/20: €159 million) with a margin1 of 14.9% (Q4/20: 15.9%). EBIT growth was influenced by the exceptionally high prior-year base. In addition, higher costs for personnel, personal protective equipment and selected medical products, among others, had a negative impact.
In FY/21, EBIT1 of Helios Spain increased by 22% (24% in constant currency) to €514 million (FY/20: €420 million) with a margin1 of 12.8% (FY/20: 12.1%).
Net income1,2 increased by 1% to €227 million (Q4/20: €225 million). In FY/21, net income1,2 increased by 9% to €728 million (FY/20: €666 million).
Operating cash flow increased to €609 million (Q4/20: €434 million) with a margin of 21.1% (Q4/20: 16.5%) driven by the positive business development as well as stringent working capital management. In FY/21, operating cash flow increased to €1,204 million (FY/20: €1,149 million) with a margin of 11.1% (FY/20: 11.7%).
For FY/22, Fresenius Helios expects organic sales3 growth in a low- to mid-single-digit percentage range and constant currency EBIT4 growth in a mid-single-digit percentage range. Both sales and EBIT outlook include expected COVID-19 effects.
The Eugin Group contributed €133 million to sales and €19 million EBIT in 2021, with first-time consolidation effective April 1, 2021. Starting Q1/22, sales and EBIT of the Eugin Group will be reported under “Fertility Services” on a quarterly basis.
1 Before special items
2 Net income attributable to shareholders of Fresenius SE & Co. KGaA
3 FY/21 base: €10,891 million
4 FY/21 base: €1,127 million, FY/22 before special items
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Fresenius Vamed
Fresenius Vamed manages projects and provides services for hospitals and other health care facilities worldwide and is a leading post-acute care provider in Central Europe. The portfolio ranges along the entire value chain: from project development, planning, and turnkey construction, via maintenance and technical management to total operational management.
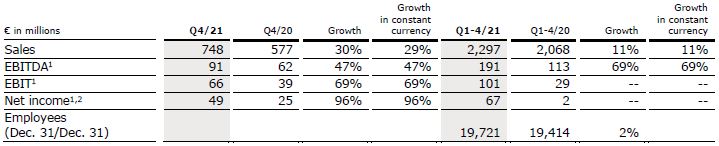
- Strong finish to the year with excellent organic sales and EBIT growth
- Project business recovering - back to the typical phasing with a strong Q4
- Rehabilitation business developing steadily despite continuous COVID-19 impact; technical service business remains robust
Sales increased by 30% (29% in constant currency) to €748 million (Q4/20: €577 million). Organic growth was 29%. In FY/21, sales increased by 11% (11% in constant currency) to €2,297 million (FY/20: €2,068 million). Organic growth was 11%.
Sales in the service business increased by 12% to €415 million (Q4/20: €372 million). Sales in the project business increased by 62% to €333 million (Q4/20: €205 million), driven by the good operating performance across all regions. In FY/21, sales in the service business increased by 10% to €1,580 million (FY/20: €1,435 million). Sales in the project business increased by 13% to €717 million (FY/20: €633 million).
1 Before special items
2 Net income attributable to shareholders of VAMED AG
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
EBIT1 increased by 69% (69% in constant currency) to €66 million (Q4/20: €39 million) with a margin1 of 8.8% (Q4/20: 6.8%). This significant recovery is due to the good business performance in all regions. In FY/21, EBIT1 more than tripled (248% in constant currency) to €101 million (FY/20: €29 million) with a margin1 of 4.4% (FY/20: 1.4%).
Net income1,2 increased to €49 million (Q4/20: €25 million). In FY/21, net income1,2 increased to €67 million (FY/20: €2 million).
Order intake was €319 million in Q4/21 (Q4/20: €648 million) and €1,290 million in FY/21 (FY/20: €1,010 million). As of December 31, 2021, order backlog was at €3,473 million (December 31, 2020: €3,055 million).
Operating cash flow increased to €128 million (Q4/20: €74 million) with a margin of 17.1% (Q4/20: 12.8%) mainly due to an improved working capital development. In FY/21, operating cash flow increased to €151 million (FY/20: €78 million) with a margin of 6.6% (FY/20: 3.8%).
For FY/22, Fresenius Vamed expects organic sales3 growth in a high-single to low-double-digit percentage range and constant currency EBIT4 to return to absolute pre-COVID-19 levels (FY/19: €134 million). Both sales and EBIT outlook include expected negative COVID-19 effects.
1 Before special items
2 Net income attributable to shareholders of VAMED AG
3 FY/21 base: €2,297 million
4 FY/21 base: €101 million; FY/22 before special items
For a detailed overview of special items please see the reconciliation tables on pages 21-25 in the PDF.
Press Conference
As part of the publication of the results for FY 2021, a press conference will be held on February 22, 2022 at 10 a.m. CET. You are cordially invited to follow the press conference in a live broadcast over the Internet at https://www.fresenius.com/media-calendar. Following the press conference, a replay will be available on our website.
For additional information on the performance indicators used please refer to our website https://www.fresenius.com/alternative-performance-measures.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g. changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius does not undertake any responsibility to update the forward-looking statements in this release.
- Higher than anticipated COVID-19-related excess mortality, but declining throughout the quarter
- Earnings development affected by ongoing significantly elevated labor costs compounded by effects from Omicron in Health Care Services and by increased material and logistics costs in Health Care Products
- Earnings development in EMEA additionally impacted by the war in Ukraine
- Financial targets for FY 2022 confirmed
Rice Powell, Chief Executive Officer of Fresenius Medical Care, said: “When we see what is happening in Ukraine, it is again above all the human tragedy that leaves us deeply saddened. I am incredibly thankful and proud of all who continue to work tirelessly to ensure patient care and the holding up of our local operations under these outstandingly difficult circumstances. Although it is difficult to talk about numbers with these images in mind, I have to say that in addition, Omicron has affected the quarter heavily. This resulted in high excess mortality among our patients and significantly elevated labor costs in the U.S. to manage isolation clinics and shifts. We were able to compensate this and delivered the quarter in line with our expectations. Based on a strong decline in excess mortality in February and March, we confirm our financial targets for 2022.”
Higher than expected COVID-19-related excess mortality at the beginning of the year
COVID-19-related excess mortality among Fresenius Medical Care’s patients amounted to approximately 2,310 in the first quarter of 2022 (Q1 2021: ~3,200; Q2 2021: ~1,900;
Q3 2021: ~2,900; Q4 2021: ~2,0003). It significantly declined in February and March in line with infection rates, but on a quarterly basis still exceeded the originally anticipated level. This resulted in an increased need for isolation clinics and shifts and limited the Company’s ability to mitigate the impacts from labor shortage and wage inflation in the U.S. market.
COVID-19-related excess mortality accumulated to approximately 9,000 patients over the past twelve months and to approximately 22,600 since the start of the pandemic.
The overall estimated adverse effect of accumulated COVID-19-related excess mortality on organic growth in the Health Care Services business amounted to around 290 basis points in the first quarter.
1 2021: costs related to the FME25 program; 2022: costs related to the FME25 program and impacts related to the war in Ukraine
2 Net income attributable to shareholders of Fresenius Medical Care AG & Co. KGaA
3 Historical excess mortality updated for late entries
War in Ukraine impacting business development
The war in Ukraine is affecting Fresenius Medical Cares' dialysis operations and patient care in the country itself, but also caused higher bad debt expenses in Russia and Ukraine. The direct adverse effect of the war in Ukraine amounted to EUR 22 million at operating income level in the first quarter and is treated as a special item. Fresenius Medical Care will continue to monitor closely the potential effects of the war as well as the general impact of the challenging inflationary macroeconomic environment.
First quarter earnings development in line with expectations
Revenue increased by 8% to EUR 4,548 million (+3% at constant currency, +2% organic).
Health Care Services revenue increased by 8% to EUR 3,607 million (+3% at constant currency, +1% organic). At constant currency, this was mainly driven by organic growth, which was achieved despite the adverse impact of COVID-19, the partial reversal of an accrual related to a revenue recognition adjustment for accounts receivable in legal dispute and contributions from acquisitions.
Health Care Products revenue increased by 6% to EUR 941 million (+3% at constant currency, +3% organic). Constant currency growth was mainly driven by higher sales of in-center disposables and renal pharmaceuticals. This was partially offset by lower sales of machines for chronic treatment.
Operating income decreased by 27% to EUR 348 million (-30% at constant currency), resulting in a margin of 7.6% (Q1 2021: 11.3%). Operating income excluding special items, i.e. costs incurred for FME25 and the impacts related to the war in Ukraine, declined by 15% to EUR 403 million (-19% at constant currency), resulting in a margin of 8.9% (Q1 2021: 11.3%). At constant currency, the decline was mainly due to higher labor costs, adverse COVID-19-related effects, as well as inflationary and supply chain cost increases. These effects were only partially mitigated by the partial reversal of an accrual related to a revenue recognition adjustment for accounts receivable in legal dispute.
Net income2 decreased by 37% to EUR 157 million (-39% at constant currency). Excluding special items, net income declined by 20% to EUR 200 million (-23% at constant currency), mainly due to the mentioned negative effects on operating income.
Basic earnings per share (EPS) decreased by 37% to EUR 0.54 (-39% at constant currency). EPS excluding special items declined by 20% to EUR 0.68
(-23% at constant currency).
Cash flow development
In the first quarter, Fresenius Medical Care generated EUR 159 million of operating cash flow (Q1 2021: EUR 208 million), resulting in a margin of 3.5% (Q1 2021: 4.9%). The decrease was mainly due to continued recoupment of the U.S. government’s payments received in 2020 under the CARES Act and a decrease in net income, partially offset by a favorable impact from trade accounts and other receivables.
Free cash flow4 amounted to EUR -1 million (Q1 2021: EUR 29 million) in the first quarter, resulting in a margin of 0.0% (Q1 2021: 0.7%).
Regional developments
In North America, revenue increased by 9% to EUR 3,171 million (+2% at constant currency, +0% organic). At constant currency, this was mainly driven by organic growth in the Health Care Product business and the reversal of an accrual related to a revenue recognition adjustment for accounts receivable in legal dispute. This was partially offset by the adverse COVID-19 impact on the Health Care Services business.
Operating income in North America decreased by 24% to EUR 304 million (-29% at constant currency), resulting in a margin of 9.6% (Q1 2021: 13.7%). At constant currency, the decline in operating income was mainly due to higher labor costs, the adverse impact of COVID-19, inflationary and supply chain cost increases as well as costs related to FME25. This was only partially offset by the partial reversal of an accrual related to a revenue recognition adjustment for accounts receivable in legal dispute.
Revenue in the EMEA region increased by 1% to EUR 674 million in the first quarter (+3% at constant currency, +2% organic). At constant currency, this was mainly due to organic growth in the Health Care Services business, which was achieved despite the negative impact of COVID-19.
Operating income in EMEA decreased by 23% to EUR 61 million (-19% at constant currency), resulting in a margin of 9.1% (Q1 2021: 11.9%). The decline was mainly due to the impact related to the war in Ukraine.
4 Net cash provided by / used in operating activities, after capital expenditures, before acquisitions, investments, and dividends
In Asia-Pacific, revenue increased by 8% to EUR 507 million (+4% at constant currency, +4% organic). At constant currency, this was mainly driven by organic growth in the Health Care Products business.
Operating income increased by 16% to EUR 99 million (+14% at constant currency), resulting in a margin of 19.5% (Q1 2021: 18.1%). At constant currency, this was mainly due to a gain from the sale of clinics, favorable currency transaction effects and growth in the Health Care Products business.
Latin America revenue increased by 15% to EUR 183 million (+15% at constant currency, +16% organic), mainly driven by strong organic growth in both the Health Care Services and Health Care Products business.
Operating income improved by 68% to EUR 11 million (+51% at constant currency), resulting in a margin of 6.1% (Q1 2021: 4.2%). This was mainly due to a favorable currency transaction effect, which was partially offset by inflationary cost increases.
Patients, clinics and employees
As of March 31, 2022, Fresenius Medical Care treated 343,493 patients in 4,153 dialysis clinics worldwide and had 122,635 employees (full-time equivalents) globally, compared to 124,995 employees as of March 31, 2021.
Outlook
Based on the results for the first quarter, which were in line with the Company’s expectations, Fresenius Medical Care confirms its financial targets for 2022. The earnings improvement will be driven by expected business growth, PPE cost reduction and FME25 savings. The Company expects revenue and net income to grow at low to mid-single digit percentage rates in FY 2022.5
5 These targets are based on the 2021 results excluding the costs related to FME25 of EUR 49 million (for Net Income). They are based on the assumptions outlined in the Press Release on the Q4 and FY 2021 results (Feb. 22, 2022), in constant currency and exclude special items. Special items include further costs related to FME25, the impacts related to the war in Ukraine, and other effects that are unusual in nature and have not been foreseeable or not foreseeable in size or impact at the time of giving guidance.
Conference call
Fresenius Medical Care will host a conference call to discuss the results of the first quarter 2022 on May 4, 2022 at 3:30 p.m. CEST / 9:30 a.m. EDT. Details will be available on the Fresenius Medical Care website in the “Investors” section. A replay will be available shortly after the call.
Please refer to our statement of earnings included at the end of this news and to the attachments as separate PDF files for a complete overview of the results of the first quarter 2022. Our 6-K disclosure provides more details.
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to COVID-19, results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Implementation of measures as presented herein may be subject to information and consultation procedures with works councils and other employee representative bodies, as per local laws and practice. Consultation procedures may lead to changes on proposed measures.
Pagination
- Previous page
- Page 10
- Next page












