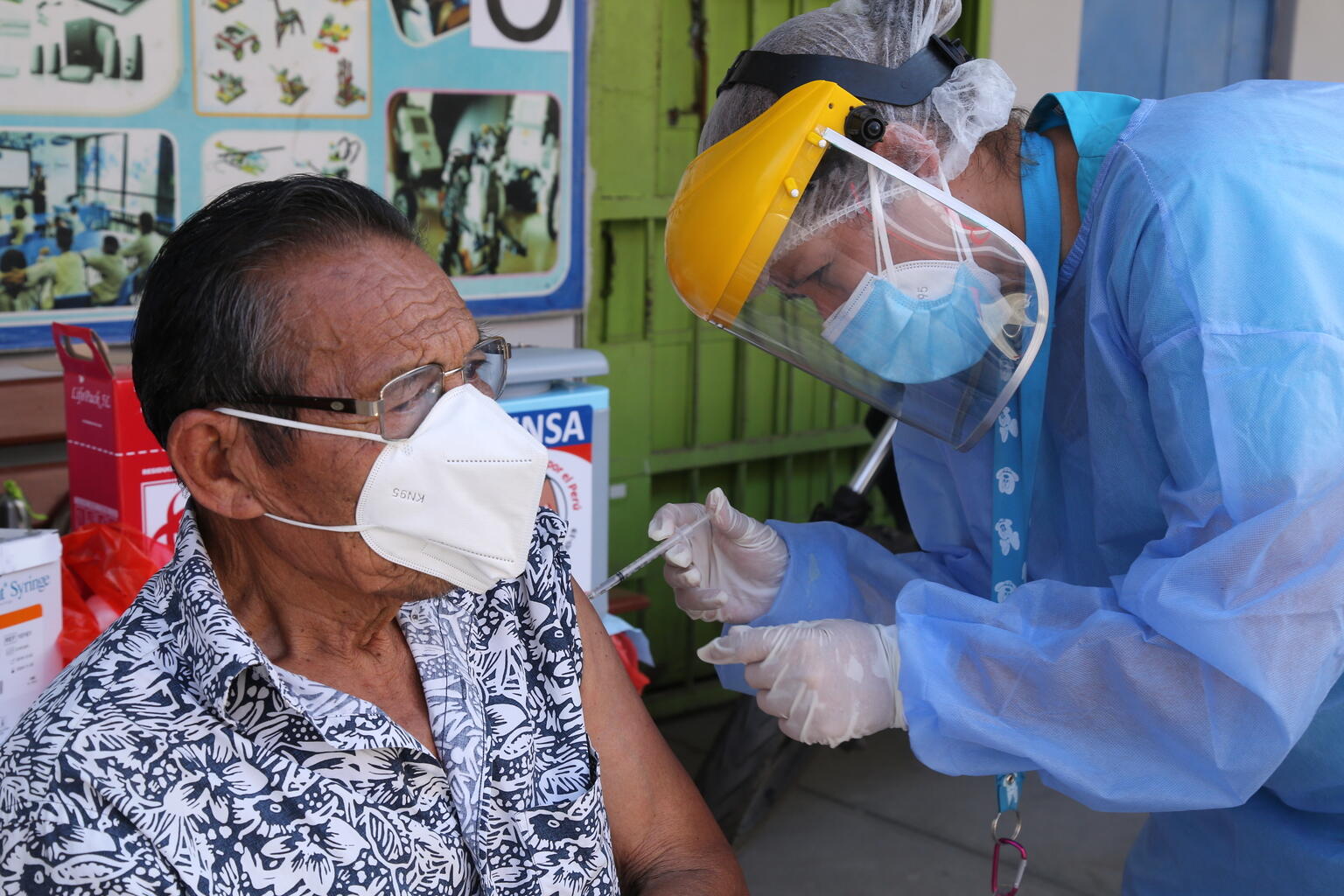
Fresenius Kabi announced today that it has received 510(k) regulatory clearance from the U.S. Food and Drug Administration (FDA) for its wireless Agilia® Connect Infusion System which includes the Agilia Volumetric Pump and the Agilia Syringe Pump with Vigilant® Software Suite-Vigilant Master Med technology. Both pumps are the first to be cleared by following TIR101 standards, which were developed by the Association for the Advancement of Medical Instrumentation (AAMI) in 2021. The product offering for hospitals and clinics enables the centralized distribution of drug libraries, warehousing of infusion data for reporting and analysis, and wireless maintenance and calibration of devices. The clearance is an important milestone for the company’s infusion therapy business in the U.S.
Fresenius Medical Care North America announced today partnerships with more than 1,000 nephrology providers in the U.S. as part of the new Kidney Care Choices (KCC) models. These partnerships comprise 20 of the 55 approved Kidney Contracting Entities (KCEs) recently announced by the Centers for Medicare and Medicaid Services (CMS). The company will help manage care for more than 50,000 individuals by providing specialized education and support services to slow the progression of kidney disease, increase preemptive transplants, and increase the prevalence of a planned start to life-sustaining treatment.
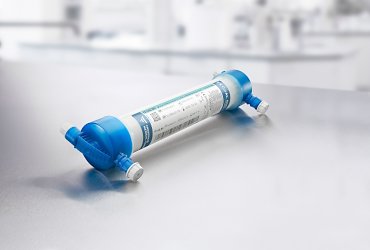
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, is launching a new dialyzer for hemodialysis, the FX CorAL®. Focal points of the new dialyzer’s development were clinical performance and hemocompatibility. The FX CorAL is already available in Switzerland and will be gradually introduced in Germany, France, Italy and the United Arab Emirates. Other countries in the company’s Europe, Middle East and Africa region will follow.
Dialysis patients often suffer from chronic inflammation caused by the accumulation of toxic substances and the regular contact of their blood with external substances. These inflammations reduce patients’ quality of life and contribute to the onset of cardiovascular diseases. The core of the new FX CorAL is its new Helixone® hydro membrane, which forms a special gel-like layer of water on the surface of the inner membrane that reduces protein adsorption while the blood is being cleaned. This is to achieve a lower induction of the immune response in the patient while maintaining high selective permeability for the removal of toxins and excess water.
Dr. Olaf Schermeier, Fresenius Medical Care’s Chief Executive Officer for Global Research & Development, said: “The well-being of our patients is at the heart of everything we do at Fresenius Medical Care. Combining clinical performance with hemocompatibility is a central element of our patient centered dialysis: It was with precisely this goal that we developed the new FX CorAL.”
Dr. Katarzyna Mazur-Hofsäß, Chief Executive Officer for Fresenius Medical Care’s Europe, Middle East and Africa region, said: “We at Fresenius Medical Care are proud that every day we help to improve the lives of people with chronic kidney disease. We work continuously to further improve treatment quality for our patients. The launch of the new FX CorAL dialyzer in the EMEA region is another important step in this direction.”
The FX CorAL is the newest dialyzer in Fresenius Medical Care’s FX class of dialyzers, which use polysulfone, a special plastic with exceptional filtering and hemocompatibility characteristics. The special structure of the dialyzer’s fibers ensures that the dialysis fluid washes evenly around each fiber, improving clearance. A lateral blood inlet port prevents the bloodline from kinking during use, enabling a more homogenous blood flow and making treatment safer. In addition, all FX-class dialyzers are steam-sterilized and, owing to their light material, environmentally friendly. After receiving the CE-mark, the FX CorAL was introduced in Fresenius Medical Care’s NephroCare clinics and has already been used in more than 3 million treatments.
In hemodialysis, the dialyzer acts as an artificial kidney and takes over vital functions of the natural organ. Blood flows through as many as 20,000 extremely fine fibers, known as capillaries, which are clustered in a plastic tube approximately 30 centimeters (12 inches) long. Metabolic toxins and excess water are filtered from the blood through these capillaries, and then flushed out of the body with dialysis fluid. Blood cells and vital proteins, however, remain in the blood. This treatment is usually administered three times a week and lasts three to six hours per treatment.
About 43 percent of all dialyzers sold worldwide come from Fresenius Medical Care’s development and production sites.
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Signs marked with ® are registered trademarks of Fresenius Medical Care in selected countries.
Fresenius Kabi and Omnicell, Inc are collaborating to provide U.S. hospitals and health systems with innovative new pharmacy technology designed to support safety and efficiency in dispensing of controlled substances in patient care areas. Omnicell has designed new cassettes for its Controlled Substance Dispenser specifically for Fresenius Kabi Simplist® MicroVault® pre-filled syringes. The new cassettes provide customers with more flexibility and enhanced options for controlled substance management. The collaboration with Omnicell is an example of the continued investment Fresenius Kabi has made in its Simplist® portfolio. The investment has resulted in more Simplist® products for customers to choose from including those that offer radio frequency identification (RFID) technology.

Fresenius Medical Care, the world’s leading provider of products and services for individuals with renal diseases, has conducted a study examining the effectiveness of the “Medical Patient Review continuous quality improvement” (MPR-CQI) program in 20 countries of the region Europe, Middle East and Africa (EMEA). The study has demonstrated, by means of mediation analysis, that improvements in medical key performance indicators (KPIs), occurring after the MPR-CQI program implementation, was associated with a significant reduction in mortality risk of about 30 percent in study participants.
For the development of the MPR-CQI program, the company used therapy data from more than 70,000 patients treated in Fresenius Medical Care’s EMEA NephroCare network to define, monitor and improve 10 KPIs – intermediate clinical endpoints – including hemoglobin, hydration status, hemodynamic status and infusion volume. The Medical Patient Review’s monthly review circles and evaluations of patients’ health indicators allow physicians to identify, prioritize and address patient’s medical needs.
“Delivering the highest quality care for people living with kidney disease is central to our mission, and data-driven insights are vital for ongoing clinical care improvement and innovation,” said Franklin W. Maddux, MD, Global Chief Medical Officer of Fresenius Medical Care. “The breadth and depth of our company’s clinical data, combined with our efforts in digital innovation and connected health, are generating a compelling evolution of clinical results.”
The quality performance of Fresenius Medical Care’s clinical governance strategy has been evaluated in an historical cohort study based on the company's own EuCliD database in the EMEA region. The company has published the study results in Nephrology, Dialysis & Transplantation, the official journal of the European Renal Association/European Dialysis and Transplant Association, to make them publicly available to the professional community and support further improvements in care worldwide: https://doi.org/10.1093/ndt/gfab160
The Medical Patient Review continuous quality improvement program is based on EuCliD data – Fresenius Medical Care’s digital patient care system- and enables the company to define evaluation processes involving medical and nursing experts. It also enables a detailed analysis of patients that allows a better understanding of medical needs, while providing intensive experiences from correlating improvements in medical and economic efficiency results – to deliver feedback focused on effective and efficient care. Through its digital patient care system, Fresenius Medical Care is planning to also enable third-party clients to benefit from the MPR.
As part of its 2025 growth strategy, Fresenius Medical Care is using digital technologies and artificial intelligence (AI) to develop new forms of kidney therapy. Physicians in more than 100 clinics in the company’s network can already use an AI-based algorithm that can help to improve renal anemia management while reducing drug-related costs. Fresenius Medical Care is planning to enrich the Medical Patient Review process by the MPR Advanced Benchmarking System which is based on an ensemble of 22 AI-based models that are designed to help detect best clinical practices, pinpoint high-performing centers beyond case-mix differences, discover emerging medical needs and set realistic margins of improvement for each intermediate clinical endpoint in all clinics.
The MPR Advanced Benchmarking System will be piloted starting this fall in NephroCare clinics in one EMEA country. Additional AI-based products for the management of non-dialysis dependent chronic kidney disease, vascular access care, home dialysis therapies and intradialytic complications are now being developed.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, has donated 250,000 euros to UNICEF, the United Nations Children's Fund, to mark the company’s 25th anniversary. UNICEF will use the donation to support the COVAX vaccination initiative to fight the COVID-19 pandemic in about 140 countries. UNICEF delivers COVID-19 vaccines and other equipment around the world. With 250,000 euros UNICEF is able, for example, to protect almost 70,000 teachers or medical personnel from a COVID-19 infection. With every health worker who is protected from the virus the care of numerous children can be ensured. With every vaccinated teacher, more and more children can return to school.
Rice Powell, CEO of Fresenius Medical Care, presented a symbolic check at a virtual ceremony. “As a company in the healthcare branch, we bear a special responsibility to our patients, to our employees and as a member of our society as well,” he said. “In this anniversary year, we must do everything to defeat the global corona pandemic. We are doing this by various means. Our donation to UNICEF is targeted to protect the health of children in less developed and in developing countries. This lives up to our values and our mission for the past 25 years to maintain life and improve the quality of life.”
As the world’s largest single buyer of vaccines, UNICEF has unique experience, gained over many years, in purchasing and delivering goods to children in need. Working on behalf of nearly 100 countries, UNICEF procures more than 2 billion vaccine doses for routine vaccinations and outbreaks of diseases every year. Over the past 20 years, the organization helped provide more than 760 million children with life-saving vaccines.
Christian Schneider, Executive Director of UNICEF Germany said: “The impact of the COVID-19 pandemic threatens children around the world. Their access to health facilities is compromised, many still cannot go to school. Also, violence against children is increasing. The COVAX vaccine initiative plays a decisive role in fighting the pandemic and reducing its consequences, especially in the lower-income regions of the world. The pandemic is not over for anyone until it is over for everyone. To that end, UNICEF provides its unique and longstanding expertise in vaccine procurement and logistics. But we cannot meet this challenge alone. We therefore thank Fresenius Medical Care for their generous support.”
COVAX is a global initiative in which the vaccination alliance Gavi, the World Health Organization (WHO), UNICEF and the Coalition for Epidemic Preparedness Innovations (CEPI) are working together to facilitate the equitable global distribution of COVID-19 vaccines.
From the beginning of the COVID-19 pandemic, Fresenius Medical Care has been intensely engaged on a variety of fronts. In addition to sweeping measures to protect against infection and to ensure continuous operation of our dialysis centers, the company opened its facilities in several countries to allow vaccination of patients and, in some cases, the general public.
Fresenius Medical Care is the world's leading provider of products and services for individuals with renal diseases of which around 3.7 million patients worldwide regularly undergo dialysis treatment. Through its network of more than 4,100 dialysis clinics, Fresenius Medical Care provides dialysis treatments for approximately 346,000 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with its core business, the Renal Care Continuum, the company focuses on expanding in complementary areas and in the field of critical care. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
UNICEF is the United Nations Children's Fund. Every child in the world has the right to a childhood – UNICEF is there to make this right a reality. UNICEF was founded in 1946 to help children in devastated Europe after the Second World War. Today, UNICEF works in 190 countries worldwide to ensure that every child can develop healthily, grow up in a protected environment and go to school – regardless of religion, skin colour or origin.
Together with many supporters and partners, UNICEF for example provides every third child worldwide with vaccines, trains teachers, equips schools and advocates for effective child protection laws.
For more information go to: www.unicef.org
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, announced publication of its 2021 Global Annual Medical Report. Titled “Embracing the Complexity of Global Healthcare,” the report focuses on addressing healthcare complexities by driving meaningful advances in kidney disease care.
“The volume of thought leadership, ideas and expertise in the report demonstrates our company’s continued leadership in kidney care, our innovation through lifelong learning and our commitment to our patient-centered mission,” said Franklin W. Maddux, MD, Global Chief Medical Officer of Fresenius Medical Care.
The 2021 Global Annual Medical Report comprises 25 chapters by more than 40 authors from across the company. Six different sections each focus on a core theme of Fresenius Medical Care’s Clinical & Quality Agenda. An additional COVID-19 section is dedicated to the company’s ongoing efforts to address the pandemic.
The core themes are:
- Cardiovascular Health
- Precision Medicine
- Communication and Medical Education
- Global Research
- Patient-Centered Care
- Innovation and Transformation
The 2021 Global Annual Medical Report is published by the Global Medical Office of Fresenius Medical Care. The full report is now available online at: https://www.freseniusmedicalcare.com/en/about-us/sustainability/medical-responsibility/
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Kabi has reached a milestone on the road to approval for another biosimilar. MSB11456, a tocilizumab biosimilar candidate, successfully met the primary and secondary endpoints in two consecutively conducted clinical trials. For more information, please visit the Fresenius Kabi website.
Fresenius Medical Care expands capabilities at its Global Medical Office: As part of a holistic approach to kidney therapy, the company aims to play an active role in the field of kidney transplantation. To achieve this, Fresenius Medical Care has created the position of Head of Transplantation Medicine and appointed Dr. Benjamin Hippen (49), a US citizen, to fill the post. In this role, he will lead the company’s worldwide efforts to expand access to and understanding of transplant medicine. Dr. Hippen is a distinguished general and transplant nephrologist. He previously served on the Board of Directors of InterWell Health in North America, a nephrology network in which Fresenius Medical Care participates.
Pagination
- Previous page
- Page 7
- Next page