Fresenius Kabi’s tocilizumab biosimilar candidate, MSB11456, has received a positive recommendation for a marketing authorization from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP). MSB11456 becomes the first tocilizumab biosimilar candidate to be granted a positive opinion by the CHMP for the treatment of several autoimmune diseases. This achievement marks a significant milestone in Fresenius Kabi’s Vision 2026 growth strategy.
For more information, please see the website of Fresenius Kabi.
Fresenius Kabi announced today that its biosimilar candidate FKS518, a denosumab biosimilar candidate for the US reference product Prolia®*, successfully met its primary and secondary objectives in a recently conducted clinical trial on pharmacokinetic similarity. For more information, please see the website of Fresenius Kabi.
*Prolia® is a registered trademark of Amgen Inc.
Fresenius Kabi today announced the immediate availability of two drugs in the United States. The first product, Ganirelix Acetate Injection, a generic fertility drug is part of the company’s expansion in women’s health. Ganirelix Acetate Injection is indicated for the inhibition of premature surges in luteinizing hormone, a chemical in the body that triggers reproductive processes such as ovulation. The second product is fentanyl citrate in a Simplist® ready-to-administer prefilled syringe presentation. The product is the only 100 mcg per 2 mL presentation available on the U.S. market in a manufacturer-prepared prefilled syringe.
Fresenius Kabi announced today the launch of its citrate-free biosimilar Idacio®* (adalimumab-aacf) for use in the treatment of chronic autoimmune diseases, in the U.S. Idacio® (adalimumab-aacf) is a tumor necrosis factor (TNF) blocker and a biosimilar to Humira®** (adalimumab). Idacio® is Fresenius Kabi’s second biosimilar launched in the U.S. and another key milestone in the company’s Vision 2026 growth strategy to broaden its biopharma global reach.
* Idacio® is a registered trademark of Fresenius Kabi Deutschland GmbH in selected countries
**Humira® is a registered trademark of AbbVie, Inc.
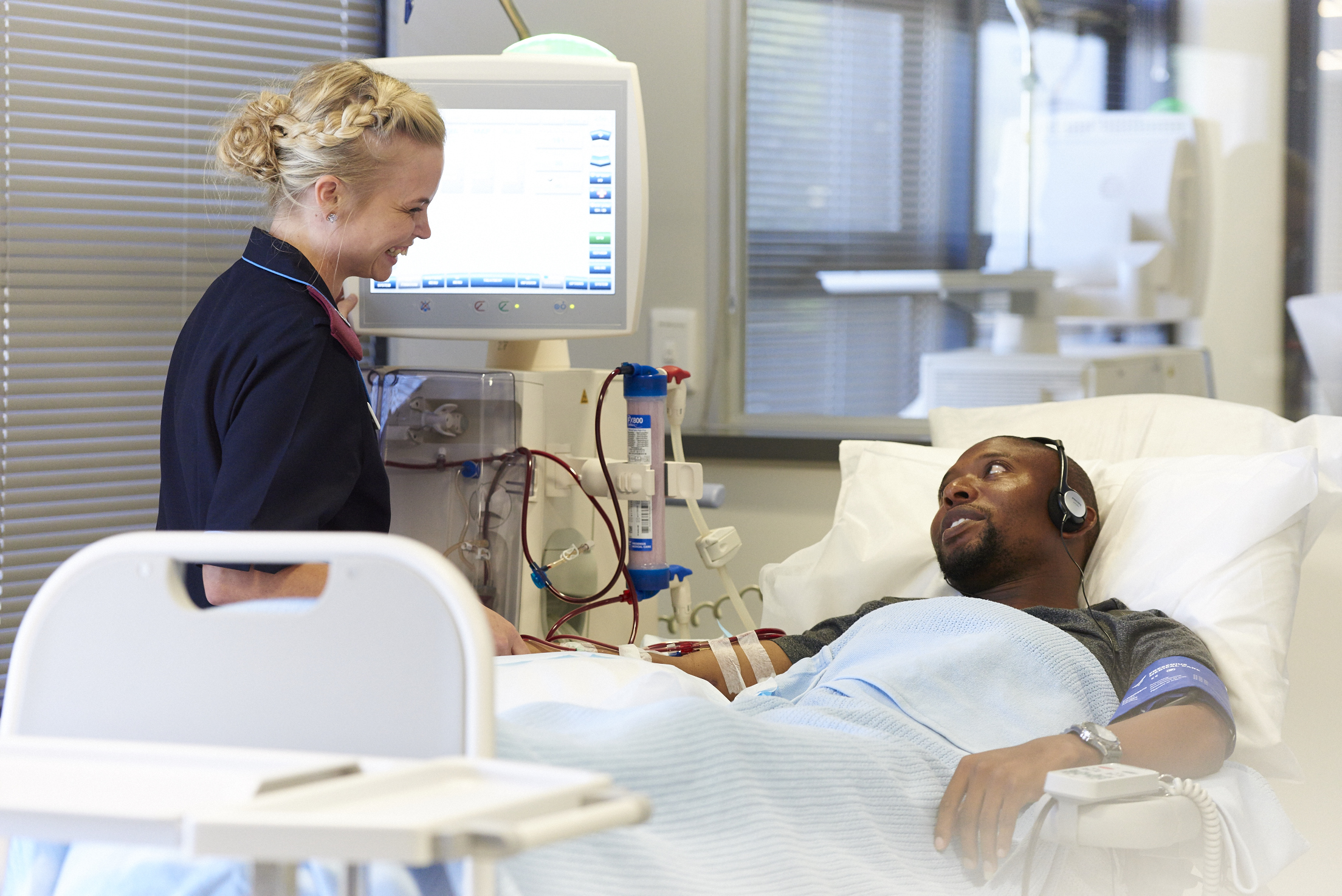
- The CONVINCE Trial study compares high-dose hemodiafiltration with standard, high-flux hemodialysis
- Study revealed a 23% decrease in mortality rates for patients treated with high-volume hemodiafiltration compared to those treated with more commonly used high-flux hemodialysis
- Exploration of methods to increase adoption of hemodiafiltration (HDF) making this therapeutic option accessible to patients
Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, participated in groundbreaking research that demonstrates that the mortality rate among kidney failure patients can be significantly reduced through the utilization of high-dose hemodiafiltration technology. Conducted by the CONVINCE consortium and led by the University Medical Center Utrecht, this international, randomized controlled trial marks a crucial milestone in comparing high-dose hemodiafiltration with standard, high-flux hemodialysis.
The study's findings reveal that patients treated with high-dose hemodiafiltration experienced a remarkable 23% decrease in mortality rates compared to those treated with the more commonly used high-flux hemodialysis. Hemodialysis represents the most prevalent form of dialysis for treating kidney failure. While techniques have improved over time, conventional high-flux hemodialysis primarily employs diffusion to remove small molecules and fluid from the blood. In contrast, high-dose hemodiafiltration incorporates both diffusion and convection techniques to eliminate larger molecules and effectively manage fluid replacement through convection.
Frank Maddux, MD, Chief Global Medical Officer of Fresenius Medical Care, expressed enthusiasm over the study's results, stating, "This meticulously designed and executed clinical study unquestionably demonstrates the efficacy of high volume hemodiafiltration for a significant portion of kidney failure patients. These findings have the potential to prompt significant changes in the standard treatment approach and, most importantly, contribute to reducing mortality rates among this vulnerable population in need of kidney replacement therapy."
Lead investigator Professor Peter Blankestijn from UMC Utrecht commented, "Our results unequivocally demonstrate the survival benefits of utilizing hemodiafiltration over hemodialysis in the treatment of kidney failure, akin to a remarkable 23% reduction in all-cause mortality. I am optimistic that hemodiafiltration can become the new standard of care.”
Chronic kidney disease poses a pressing global health challenge, affecting an estimated 830 million individuals worldwide, with nearly four million people reliant on dialysis. When the kidneys can no longer perform their vital functions, dialysis is employed to cleanse the blood by eliminating waste products and regulating fluid volume in the body.
The trial encompassed a total of 1,360 patients across 61 centers in eight European countries and followed up for a median of 30 months. Of these, 683 patients received high-dose hemodiafiltration and 677 received high-flux hemodialysis three times a week. The rate of mortality was 7.1 deaths per 100 patient-years in the group randomized to hemodiafiltration compared to 9.2 deaths per 100 patient-years in the hemodialysis group, corresponding to a relative reduction of 23%.
The CONVINCE trial was spearheaded by researchers from UMC Utrecht in collaboration with University College London (UCL), Charité Universitätsmedizin Berlin, University of Bari, The George Institute for Global Health, Imperial College London, and dialysis providers Fresenius Medical Care, Diaverum, and B. Braun Avitum.
While hemodialysis serves as the standard treatment in most countries, hemodiafiltration remains underutilized in certain regions and is yet to be adopted in places like the U.S. Fresenius Medical Care has taken the lead in developing machines that facilitate online fluid generation and innovative techniques for delivering hemodiafiltration.
"Fresenius Medical Care has a rich history of driving innovation in renal care, aimed at improving the lives of kidney disease patients worldwide," emphasized Frank Maddux, MD. "As pioneers in dialysis and membrane technologies, we will continue to explore methods that promote the adoption of hemodiafiltration, making this crucial therapeutic option more readily accessible to the patients we serve."
Disclaimer:
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to various factors, including, but not limited to, changes in business, economic and competitive conditions, legal changes, regulatory approvals, impacts related to the COVID-19 pandemic results of clinical studies, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
__________
Notes to Editors:
Disclaimer: The information in this document is provided as is and no guarantee or warranty is given that the information is fit for any particular purpose. The user thereof uses the information at its sole risk and liability. The opinions expressed in the document are of the authors only and in no way reflect the European Commission’s opinions.
The CONVINCE study was exclusively supported by the European Commission Research & Innovation, Horizon 2020, Call H2020-SC1-2016-2017 under the topic SC1-PM-10-2017: Comparing the effectiveness of existing healthcare interventions in the adult population (grant no 754803).
Fresenius Kabi expands its portfolio of critical care medicines by launching Vasopressin Injection, USP, a generic equivalent to Vasostrict®* in the U.S.
Fresenius Kabi Vasopressin is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
* Vasostrict® is a registered trademark of Par Pharmaceutical.
Fresenius Kabi’s Biosimilar Stimufend® (pegfilgrastim-fpgk) is now available from Fresenius Kabi in the United States. Stimufend® was approved by the U.S. Food and Drug Administration (FDA) in September 2022 for use in patients with non-myeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia. It is Fresenius Kabi’s first U.S. biosimilar launch. The expansion of the company’s global biosimilars portfolio with a focus on oncology and immunology is an important milestone in its Vision 2026 growth strategy.
* Stimufend® (pegfilgrastim-fpgk) is a registered trademark of Fresenius Kabi Deutschland GmbH in selected countries. Stimufend is a pegfilgrastim biosimilar medicine of Neulasta®, which is a registered trademark of Amgen Inc.
Fresenius Kabi’s innovative Ivenix Infusion System is recognized by KLAS Research in a Performance Insights First Look article. This report shares very positive customer experiences from health care organizations currently using the Ivenix Infusion System in the U.S. Ivenix, acquired in 2022 by Fresenius Kabi, offers a technologically advanced infusion system, including a large-volume pump, infusion management, software tools, applications, and analytics to inform care, advance efficiency, reduce infusion-related errors, and drive down the total cost of ownership.
Fresenius Kabi expands its portfolio of critical care medicine by launching Lacosamide Injection, USP, a generic equivalent to VIMPAT® in the U.S.
Fresenius Kabi Lacosamide Injection, USP is an approved treatment option for partial-onset seizures in patients 17 years of age and older and is available in the U.S. beginning today.
* VIMPAT® is a registered trademark of UCB, Inc.
The U.S. Food and Drug Administration (FDA) has approved Fresenius Kabi’s biosimilar Idacio®, a citrate-free formulation of adalimumab. Idacio® (adalimumab) is approved for use in the treatment of chronic autoimmune diseases for all eligible indications of the reference product, Humira®**.
With Idacio Fresenius Kabi expands its U.S. biosimilars portfolio focused on immunology and oncology. It is the company’s second approved biosimilar in the U.S. launching from July 2023 in accordance with the relevant patent settlement agreement with Abbvie.
* Idacio® is a registered trademark of Fresenius Kabi Deutschland GmbH in selected countries
**Humira® is a registered trademark of AbbVie, Inc.
Pagination
- Previous page
- Page 5
- Next page



