Fresenius Medical Care Ventures, the venture capital unit of Fresenius Medical Care, announced today that it has invested $2 million in the Series B financing round for Vectorious Medical Technologies (“Vectorious”). The Israeli medical device company raised more than $10 million in this financing round, including a recent grant from the European Union’s Horizon 2020 flagship research and development program.
Vectorious has developed the V-LAP, a first of its kind in-heart microcomputer for left-atrial pressure monitoring. Data from this device is transmitted wirelessly to clinicians, enabling heart failure patients to be managed effectively before their disease advances.
The Series B financing will accelerate Vectorious’ ongoing R&D programs, as well as CE Mark and FDA regulatory approval initiatives.
“This first investment we have made outside of the United States demonstrates our confidence in the great innovative potential for medical products in Israel. Based on the pioneering nature of Vectorious’ technology for managing chronic heart failure patients’ care, we see this as a compelling early stage investment opportunity with tremendous potential,” said Florian Jehle, Managing Director of Fresenius Medical Care Ventures and Senior Vice President Global R&D at Fresenius Medical Care.
About Fresenius Medical Care Ventures GmbH
Fresenius Medical Care Ventures was established in 2016 to invest in start-ups and early-stage companies in the healthcare sector. Its investments are targeted to support Fresenius Medical Care’s corporate strategy of growing continuously in the company’s core dialysis business, expanding into new business areas and improving the health of all Fresenius patients. Fresenius Medical Care Ventures complements other corporate innovation initiatives by focusing on early-stage external innovation. For more information, visit www.fmcv.com.
About Vectorious Medical Technologies
Vectorious Medical Technologies has developed an implantable microcomputer-based system that enables optimal management of heart failure patients using direct, daily left-atrial pressure (LAP) measurements. The system’s technological infrastructure is based on use of the first and only sensory implant in the world that is miniature, battery-less and wireless, and that can communicate accurate readings from the heart. The company was founded in 2011 at the RadBioMed incubator by Oren Goldshtein, Dr. Eyal Orion and Roni Weinstein. Vectorious has 15 employees at its offices in Ramat Hahayal in Tel Aviv and at the Cleveland Clinic.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3.2 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,790 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 322,253 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with its core business, the company provides related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS). For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Helios will take responsibility for practical instruction as part of the first worldwide digitalized medical studies program. The new course of study meets European certification standards and will start in the winter semester 2018/2019 at the EDU College of Medicine, Malta. Theoretical components of a bachelor’s and follow-on master’s program will be available solely via an online platform. Students will be networked into learning communities taught by faculty. About one-third of the course is practical instruction that will take place in hospitals operated by Fresenius Helios in Germany. The program initially will be limited to 75 students. The long-term goal is up to 3,000 students.
Jiménez Díaz Foundation University Hospital in Madrid has again been awarded the EFQM Recognized for Excellence award with the maximum five stars, this time also receiving the highest rating of any hospital in Spain. The 667-bed facility earned more than 650 points on the rating scale used by the European Foundation for Quality Management during a comprehensive management and process evaluation earlier this year. In the award presentation, the EFQM cited Jiménez Díaz Foundation University Hospital’s intensive focus on patient care, as well as its continuous improvements in treatments and the use of medical technology. This leading teaching hospital is part of the Quirónsalud group, the Spanish unit of Fresenius Helios.
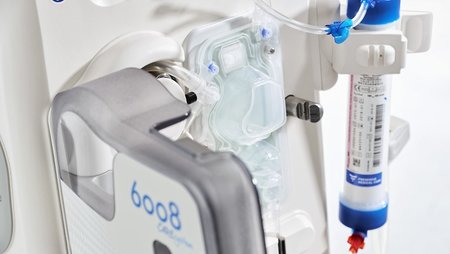
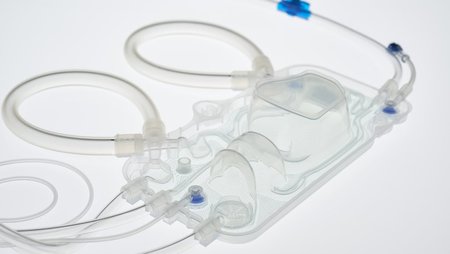
Fresenius Medical Care, the world’s largest provider of dialysis products and services, has won the Red Dot Award: Product Design for the company’s 6008 CAREset. This advanced all-in-one disposable with pre-connected bloodlines combines with the 6008 dialysis machine to form the 6008 CAREsystem. The 6008 CAREset was developed for use in all hemodialysis treatments, including Fresenius Medical Care’s advanced HighVolumeHDF dialysis therapy.
The Red Dot Award for outstanding international product design has been presented annually since 1955 by the Design Center North Rhine-Westphalia in Essen, Germany. More than 6,300 products from companies in 59 countries were entered into this year’s competition.
“The 6008 CAREsystem stands for reduced complexity, and minimizes the number of handling steps during dialysis,” said Dr. Olaf Schermeier, Fresenius Medical Care’s Chief Executive Officer for Global Research and Development. “The innovative design of the 6008 CAREset makes a key contribution to this. Because the bloodlines are pre-connected, the medical staff has more time to provide direct patient care. We are delighted about the recognition that this internationally renowned award will bring.”
Handling steps are reduced not only during dialysis, but during the set-up and disconnection phases before and after each treatment. The 6008 CAREset is simply inserted into the dialysis machine, eliminating the need to manually connect the bloodlines: This limits potential connection errors, which makes treatments safer. And in addition to being more cost efficient, the reduced amount of waste generated during a treatment makes the 6008 CAREsystem environmentally friendly.
Dialysis machines, bloodline systems and dialyzers – the latter often dubbed “artificial kidneys” because this is where the blood is actually cleaned – are the most important products for treating chronic kidney disease. During treatment, the dialysis machine pumps the patient’s blood through bloodlines, monitors its circulation through the dialyzer, and adds anti-coagulants. Treatments are generally carried out three times weekly, and take between three and six hours each.
About half of all dialysis machines and dialyzers sold worldwide are produced by Fresenius Medical Care.
More information about the Red Dot Award is available at https://en.red-dot.org.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3.2 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,752 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 320,960 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Helios has presented the new Helios Science Prize for outstanding research by employees that contributes to improved treatment quality. The three winners all work at hospitals in Germany and were honored for their work in different areas of medicine. The prize, which comes with a cash award, went to Dr. Jitka Veldema of Helios Hospital Kipfenberg (for her work on stroke rehabilitation), Prof. Dr. Alexander Kreuter of Helios St. Elisabeth Hospital in Oberhausen (cancer diagnosis in HIV patients), and Dr. Patrick Weil of Helios University Hospital in Wuppertal (testicular cancer therapy).
Unicyte AG, a pioneering leader in human liver stem cells and nano-extracellular vesicles, announced today the appointment of Prof. Jonathan Knowles to its Scientific Advisory Board.
The board works closely with Unicyte’s management team to accelerate the company’s regenerative stem cell and extracellular vesicles programs for treating diabetes, non-alcoholic fatty liver disease, diabetic nephropathy and cancer. In addition, it provides scientific advice for the collaboration between Unicyte and Italy’s University of Turin in order to foster innovation and new research programs.
"We are excited to welcome Prof. Jonathan Knowles to our Scientific Advisory Board, as he has not only the scientific expertise but also the experience of developing highly innovative technologies in both pharma and biotech companies," said Florian Jehle, CEO of Unicyte and Vice President, Technology & Innovation Management within Research & Development at Fresenius Medical Care.
Jonathan Knowles is Chairman of the Board of Directors of Immunocore Ltd. and of the Access Committee of Genomics England. He is a Visiting Professor of Translational Medicine at the University of Oxford and formerly a distinguished Professor of Personalized Medicine at the University of Helsinki and at EPFL Lausanne. As Head of Research from 1997 to 2009 he had oversight of Research and Development for the Roche Group. He also served as a director on the boards of Genentech and of Chugai Pharmaceuticals, and from 1987 to 1997 was Director of the Glaxo Institute for Molecular Biology. Jonathan Knowles holds a 1st class honors degree in Biology from the University of East Anglia in England and a PhD in Molecular Genetics from the University of Edinburgh in Scotland.
Jonathan Knowles said: “I am excited to join Unicyte’s Scientific Advisory Board and to support the company as it is anticipating first partnerships for commercialization. Unicyte has a unique adult stem cell platform that overcomes the cost constraints of many alternative approaches and has significant market potential, especially in large indications such as diabetes. Also, I am very interested in the therapeutic potential of extracellular vesicles, and Unicyte has a lead in this area.”
Unicyte originated from the long-standing research collaboration between Fresenius Medical Care, the world's leading provider of products and services for individuals with renal diseases, and Prof. Giovanni Camussi, a leading expert in nano-extracellular vesicles and stem cells at the University of Turin. Now an independent affiliate of Fresenius Medical Care, Unicyte has a broad preclinical pipeline focusing on kidney and liver disorders, diabetes and oncology, and will work with partners when needed to advance these therapeutic programs.
Dr. Daniel Gau, Unicyte’s Head of Business Development, said: “Prof. Knowles will make an outstanding and valued addition to our Scientific Advisory Board, helping Unicyte to identify new areas of focus and potentially disruptive therapies for the benefit of our patients.”
Unicyte AG is a preclinical stage regenerative medicine company with a focus on kidney and liver disorders, diabetes and oncology. Unicyte evolved from a long-term research collaboration of Italy’s University of Turin and Fresenius Medical Care. Unicyte is headquartered in Oberdorf NW, Switzerland, and is an independent affiliate of Fresenius Medical Care, the world's largest provider of products and services for people with chronic kidney failure. For more information, visit Unicyte’s website at www.unicyte.ch.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,714 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 317,792 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS). For more information visit the Company’s website at www.freseniusmedicalcare.com.
The Molecular Biotechnology Center (MBC) at the University of Turin, active since September 2006, has the main objective to bring together investigators with different scientific backgrounds to facilitate an interdisciplinary approach to biomedical research. The Center is actively involved in biotechnological research in the field of biomedical sciences, with specific focus on the study of the molecular mechanisms at the basis of physiopathological processes that have a significant impact on human health, such as cardiovascular diseases, inflammation, cancer and stem cell biology. These research efforts are mainly based on the development of the most advanced molecular imaging technology, bioinformatic analysis and the generation of mouse and zebrafish models. For more information, visit www.mbc.unito.it/en.
Fresenius Kabi has submitted a Marketing Authorization Application to the European Medicines Agency for its adalimumab biosimilar candidate of Humira®. The application is the first biosimilar regulatory filing for Fresenius Kabi. Adalimumab is approved in the EU for use in the treatment of chronic inflammatory autoimmune conditions, including different types of arthritis or Crohn's disease.
Fresenius Medical Care, the world’s largest provider of dialysis products and services, announced today the results from the first performance year from its End Stage Renal Disease Seamless Care Organizations (ESCOs). The results, which cover the period from October 2015 through December 2016, show improved health outcomes for patients receiving care coordination through the ESCOs. This success was validated by an independent report from Lewin Group, which showed a nearly nine percent decrease in hospitalization rates for these patients during the same time. As a result, Fresenius Medical Care ESCOs together generated more than $43 million in gross savings, an average 5.47% reduction in expenditures per patient, with all six of its first-year ESCOs exceeding the shared savings benchmark.
Fresenius Medical Care’s ESCO programs were established through an agreement with the Centers for Medicare & Medicaid Services (CMS) as part of CMS’ Comprehensive End Stage Renal Disease (ESRD) Care (CEC) Demonstration Program. Launched in 2015, it is the first disease-specific shared savings program in the United States. This program was designed to identify, test and evaluate new ways to improve care and healthcare cost for Americans with ESRD.
The first-year report includes results of Fresenius Medical Care’s first six ESCOs, including 176 clinics and 386 physician partners in Philadelphia, Pa.; Charlotte, N.C.; Dallas, Texas; San Diego, Calif.; Columbia, S.C. and Chicago, Ill. In January 2017, Fresenius Medical Care added 18 new ESCOs for a total of 24, expanding to nearly 800 physician partners and giving the company the largest ESCO presence of any dialysis provider in the United States.
“Participation in the ESCO program reinforces our support of physicians and the healthcare system to improve care and reduce costs,” said Rice Powell, Chairman and CEO of Fresenius Medical Care. “We are excited about our results and pleased that CMS has provided us with the opportunity to expand our services to cover even more patients.”
CMS conducted an application process to select the participants in the ESCO program. ESCO patients, or beneficiaries, are aligned to ESCOs through a claims-based process, and maintain their full Medicare benefits as well as the freedom to choose their providers. The program uses a shared savings and losses model as a financial incentive to improve care, assessed by a baseline that looks at expenditures incurred for beneficiaries in each of the three years prior to the start of the program. In the first program year, there were 16,085 beneficiaries aligned to the thirteen participating ESCOs.
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,714 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 317,792 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the company’s website at www.freseniusmedicalcare.com.
Disclaimers
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release. The statements contained in this document are solely those of Fresenius Medical Care and do not necessarily reflect the views or policies of CMS. Fresenius Medical Care assumes responsibility for the accuracy and completeness of the information contained in this document.
Fresenius Medical Care, the world's largest provider of dialysis products and services, today announced that company researchers and clinical experts from across the globe present 77 research abstracts at the 2017 American Society of Nephrology’s (ASN) Kidney Week Symposium, the largest and most influential meeting of kidney professionals in the world.
Scheduled from October 31 to November 5, 2017, in New Orleans, Louisiana, USA, the annual symposium draws more than 13,000 physicians, scientists and healthcare professionals from over 100 countries.
“Our ability to harness the power of research across our global enterprise is one of our many competitive advantages,” said Rice Powell, Chairman and Chief Executive Officer of Fresenius Medical Care. “Staying at the forefront of science drives our innovation and is central to better clinical outcomes in our core dialysis as well as in care coordination activities.”
The scope of the company’s research and quality improvement endeavours builds on Fresenius Medical Care’s strength as the world’s largest, vertically integrated healthcare company. With a focus on chronic kidney disease (CKD), end-stage renal disease (ESRD) and adjacent medical conditions, the company’s research crosses seven general categories:
- Using science and technology to characterize and improve patient outcomes
- Driving advancements in management paradigms through value based care models
- Utilizing predictive modeling for clinical decision support and better outcomes
- Characterizing the impacts of CKD options education on outcomes and modality selection
- Leveraging coordinated care initiatives to improve patient outcomes
- Defining outcomes related to the management of bone mineral metabolism in renal disease
- Identification of the influences of social determinants of health on clinical measures
“The breadth and depth of our leadership in advancing science into clinical practice underscores our mission to improve the lives of people with kidney and chronic diseases,” said Franklin W. Maddux, MD, Chief Medical Officer and Executive Vice President of Clinical and Scientific Affairs for Fresenius Medical Care North America. “In blending patient perspectives with real world evidence and traditional research, we are able to translate science into clinical practice that ultimately improves the lives of the patients who entrust us with their care.”
Fresenius Medical Care is the world's largest provider of products and services for individuals with renal diseases of which around 3 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,690 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 315,305 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with the core business, the company focuses on expanding the range of related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the Company’s website at www.freseniusmedicalcare.com.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Vamed has opened the Institute for Gender Medicine in cooperation with the Medical University of Vienna. This new research institute, in the Lower Austria town of Gars am Kamp, will focus on the different needs of female and male patients, for example in the interpretation of disease symptoms. Fresenius Vamed will be able to use the institute’s research findings to develop tailored treatment offerings in areas such as prevention and rehabilitation. The Institute for Gender Medicine complements initiatives for individualized medical care already being carried out by the VAMED International Medical Board and its approximately 650 physicians.
Pagination
- Previous page
- Page 13
- Next page