Fresenius Helios has developed a new system for measuring patient safety, and is introducing it in all 86 Helios hospitals across Germany. The new system will complement the company’s existing quality management system and combine internationally established patient safety indicators with various indicators that Helios has developed through clinical practice. These include measurements on such indicators as false diagnoses, medication errors and patient falls. When Helios begins reporting the results next year, it will be the first German hospital operator to provide patient safety data that can be compared internationally.
The price increase for hospital services in Germany has been set at 3.66% for 2020. As it is subject to negotiations at the state level as well as individual hospital discounts, the final price increase on the individual hospital level will, however, be lower.
Fresenius Helios patients in Germany can soon start accessing letters from their doctors, test results and other medical documents over an online platform – the Helios Patients Portal. This new portal can also be used to make appointments. The confidentiality of patient data is assured through the use of two-factor authentification. All 86 of the company’s hospitals, 126 medical care centers and eight prevention centers throughout Germany are being gradually linked to the Helios Patients Portal. The company is also developing a portal for doctors.
Fresenius Medical Care, the world’s leading provider of dialysis products and services, today announced the publication of its 2019 global annual medical report. Titled “Global Insights, Local Promise: Transforming Healthcare Through Interconnected Intelligence,” it outlines how the company is harnessing the power of an increasingly interconnected world to pioneer solutions that can have a large-scale impact on patient care.
The report also explores ways to apply insights gained in different markets on a global level, and how best practices can be adapted to specific market needs.
In addition to articulating experiences in dialysis care across diverse regions, the report delves deeper into several focus areas relevant to the healthcare industry and the evolution of kidney care:
- Expanding home therapies to give dialysis patients more control of their lives and improve treatment outcomes.
- Improving transplant access through open communication and collaboration among multiple stakeholders.
- Embracing diversity and an interdisciplinary approach to improve value-based care in kidney disease.
- Developing predictive models to identify dialysis clinics that are encountering problems or in need of additional support, and,
- Clarifying and explaining the role of nutrition in the prevention and treatment of chronic kidney disease.
Dr. Frank Maddux, Global Chief Medical Officer of Fresenius Medical Care, said: “Interconnected thinking is central to our way of working at Fresenius Medical Care and it demonstrates our commitment to making a difference in the lives of patients. The people of Fresenius Medical Care, all around the world, have always been our greatest asset. Harnessing the full potential of their interconnected intelligence can boost innovation, advance medical progress, develop better therapies, and help drive the transformation of healthcare systems worldwide.”
Spectra Laboratories, a wholly-owned subsidiary of Fresenius Medical Care North America, broke ground on a 200,000-square-foot (approx. 18,500 square meters) laboratory in Southaven, Mississippi yesterday. The project is expected to create more than 300 jobs over the next three years. Spectra Laboratories offers renal-specific laboratory services, using state-of-the-art equipment, automated specimen processing, and reporting applications. At Spectra’s new build-to-suit facility, employees will conduct comprehensive testing, analysis, and reporting to ensure the best possible outcomes for patients.
Fresenius Medical Care, the world’s leading provider of dialysis services and products, plans to invest EUR 60 million in its independent affiliate Unicyte AG in a Series A financing round. Unicyte, a leading regenerative medicine company with translational programs in the field of kidney disorders and other diseases, will primarily use the capital to start clinical trials of its first product candidates in 2020 and to establish the required manufacturing processes.
Over the last four years, Unicyte has created a unique set of proprietary technology platforms of human liver stem cells (HLSCs), HLSC-derived islets and nano-Extracellular Vesicles (nEVs). nEVs are stem cell-derived particles that support communication between cells.
Unicyte succeeded in confirming the disease-modifying potential of its nEVs in various preclinical models of chronic kidney disorders. Combined results of these studies demonstrate nEVs’ efficacy and underlying mechanism of action in preventing renal fibrosis and its subsequent progression to end-stage renal disease.
“Regenerative medicine could provide highly innovative therapies to chronic kidney disease patients and is becoming increasingly important for our industry. Driving regenerative therapies not only addresses the U.S. administration’s initiative to improve the prevention of end-stage renal disease, but can help us to significantly slow the progression of kidney disease and make the most innovative therapies available to our patients,” said Rice Powell, CEO of Fresenius Medical Care.
Dr. Olaf Schermeier, Fresenius Medical Care’s CEO for Global Research and Development, said: “This continued investment in Unicyte shows our commitment to developing the best treatment options for our patients across the renal care continuum. At the same time, it puts Fresenius Medical Care at the forefront of innovation in the field of regenerative renal stem cell therapy.”
“Based on our preclinical results, the commitment of Fresenius Medical Care is a strong driver for Unicyte’s next milestone, the start of clinical development with the required organizational structure. It will also provide a solid data package for our non-kidney product candidates to seek strategic partnerships as the next step,” said Florian Jehle, CEO of Unicyte.
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
Fresenius Medical Care, the world’s largest provider of dialysis products and services, is expanding its clinical research activities. Frenova Renal Research (Frenova), previously a Fresenius Medical Care North America subsidiary, will now offer its services worldwide and will be integrated into the new Global Medical Office headed by Dr. Frank Maddux, Chief Medical Officer.
Frenova offers services for the clinical development of medicines and medical products in the field of kidney research. This offering, previously limited to North America, will now be linked with the corresponding services of Fresenius Medical Care’s Europe, Middle East and Africa (EMEA) and Latin America regions and bundled under Frenova. This will enable the company to draw on a network of more than 550 researchers at over 350 locations.
“Frenova is active at the intersection of clinical research and patient care,” said Dr. Maddux. “No other provider of clinical development services understands the medical needs of people living with kidney disease as well as we do. By making this expertise available worldwide, we will enable a faster, more efficient development of medications and other products. This is an additional, important building block for improving their quality of life.”
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA's reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.
"Give life a chance" was the motto under which Fresenius Medical Care, the world’s largest provider of dialysis products and services, informed employees in Bad Homburg yesterday about organ donation. Individuals not only need to be well informed and carefully consider whether or not they wish to make an organ donation: Above all, they need to make a decision.
"Fresenius Medical Care supports organ transplantation,” said Dr. Katarzyna Mazur-Hofsäß, Fresenius Medical Care's Chief Executive Officer for Europe, Middle East and Africa. “For our patients with chronic kidney disease, a future worth living includes hope for a transplant, and that is exactly what we are preparing our patients for in our dialysis clinics. We want to educate and inform today, because during their lifetimes many people do not make a decision about a possible donation of their organs.”
Mayor Alexander Hetjes of Bad Homburg welcomed Fresenius Medical Care’s support for organ transplantation. “I’m very happy when companies in Bad Homburg also get involved in debates about social issues,” the mayor said. “To me it’s important to talk with many people about organ donation, so that we can all be better informed when making this very personal decision.”
Two organ donor recipients reported on their experiences via video, and medical information was explained to the employees by Dr. Anja Brückel of the German Organ Transplantation Foundation. "In Germany, more than 9,000 seriously ill people are waiting for a donor organ,” Dr. Brückel said. “For them, transplantation is the only way to survive or significantly improve their quality of life. However, this is only possible if people are willing to donate their organs. That’s why it is important to address the issue of organ donation during one’s lifetime, make close relatives aware of the decision, and fill out an organ donor card.”
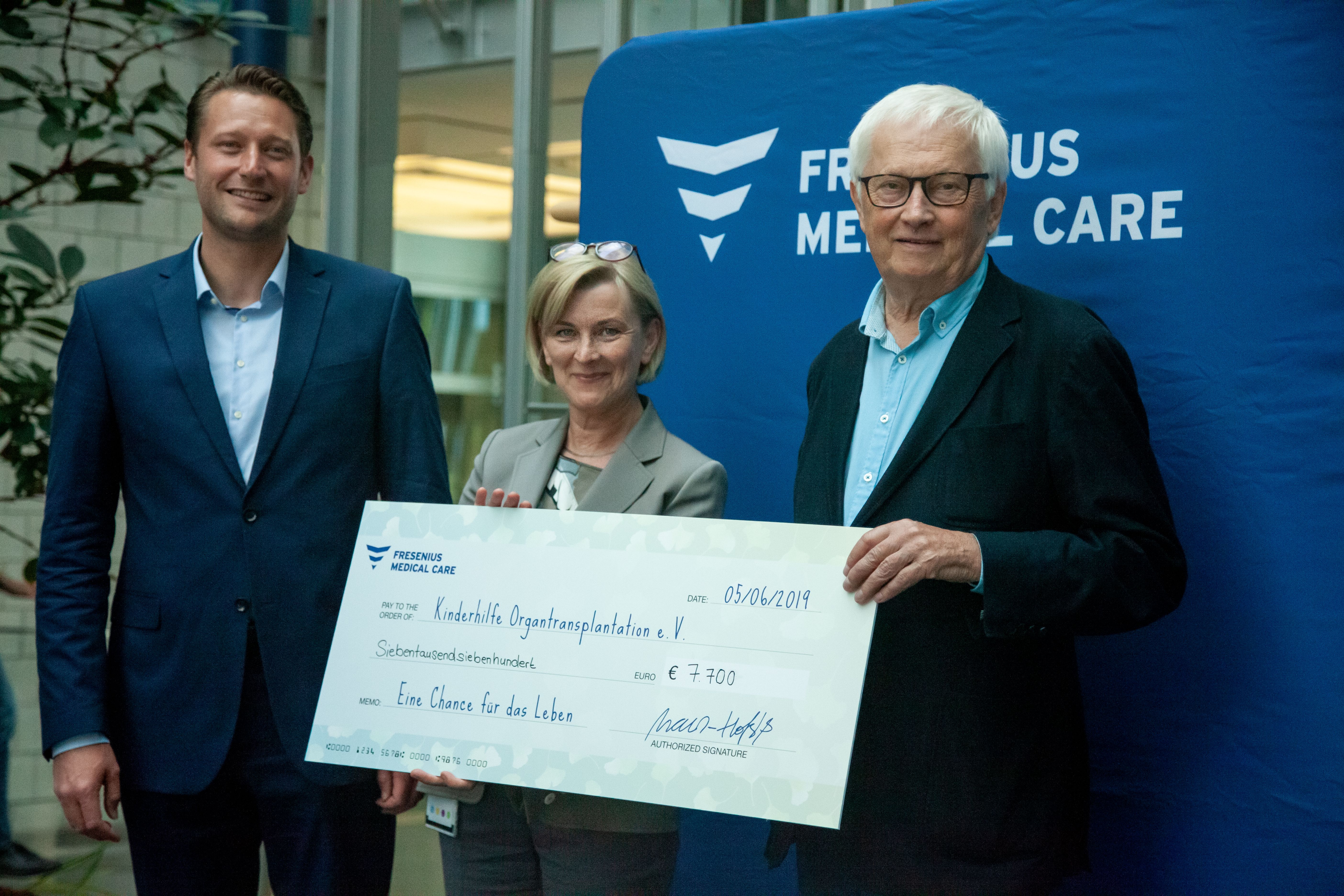
The foundation made organ donation cards available to interested employees. Mayor Hetjes, Dr. Mazur-Hofsäß and Dr. Brückel together planted a traditional gingko, the tree of life, in front of Fresenius’ new EK3 headquarters building. And after a raffle among employees raised €770 the company announced it would increase that amount 10-fold, for a total donation of €7,700 to Kinderhilfe Organtransplantation, a German charity that supports children undergoing transplants and their families.
Fresenius Kabi is launching its first biosimilar in Germany following the European Commission (EC) granting marketing authorization for IDACIO® for all indications of the reference medicine in the areas of rheumatology, gastroenterology and dermatology.
The European Commission (EC) granted Fresenius Kabi the marketing authorization for IDACIO®, an adalimumab biosimilar, for all indications of the reference medicine. With this EC approval, IDACIO® is the first approved molecule of the Fresenius Kabi biosimilars portfolio.
Pagination
- Previous page
- Page 11
- Next page